Triethylamine Density: The Ultimate Guide You Must See
The accuracy of chemical process simulations fundamentally relies on understanding properties like triethylamine density, a crucial parameter for efficient operation. Measuring refractive index, a key method to determine solution concentration, is significantly affected by the precise knowledge of triethylamine density. Consequently, the Thermo Fisher Scientific databases provide calibrated values for this vital measurement. As a result, laboratory professionals at institutions such as the National Institute of Standards and Technology (NIST) must rely on standardized measurements of triethylamine density for reliable experimental outcomes.

Image taken from the YouTube channel What Does That Mean? , from the video titled What does triethylamine mean? .
Triethylamine (TEA), a volatile, colorless liquid with a strong ammonia-like odor, is a cornerstone chemical in numerous industrial processes. Its significance stems from its role as a versatile organic base, a catalyst, and a crucial intermediate in the synthesis of a wide array of compounds. From pharmaceuticals to polymer production, TEA's influence is pervasive, making it an indispensable component in modern chemistry.
Triethylamine: A Ubiquitous Chemical Compound
TEA's widespread use can be attributed to its unique chemical properties. It readily accepts protons, making it an effective base in organic reactions. It also serves as a catalyst, accelerating reaction rates without being consumed itself.
Moreover, TEA acts as a valuable building block in the synthesis of complex molecules, enabling the creation of novel materials and pharmaceuticals. Its applications span diverse fields, including:
- Pharmaceuticals: Acting as a catalyst and intermediate in drug synthesis.
- Chemical Synthesis: Serving as a base and solvent in various reactions.
- Rubber Processing: Contributing to the production of high-quality rubber products.
- Corrosion Inhibition: Protecting metal surfaces from degradation.
The Critical Role of Density
Among TEA's many properties, its density stands out as a particularly important parameter. Density, defined as mass per unit volume, governs how TEA behaves in various applications and influences its handling, storage, and transportation. Understanding TEA's density is not merely an academic exercise; it is essential for:
- Accurate Dispensing and Metering: Ensuring precise control over reaction stoichiometry.
- Efficient Storage: Optimizing storage conditions and container selection.
- Safe Transportation: Adhering to regulatory guidelines and preventing spills.
- Quality Control: Verifying the purity and consistency of TEA batches.
Deviations in density can indicate contamination, degradation, or inconsistencies in manufacturing processes, potentially leading to compromised product quality and safety hazards.
A Comprehensive Guide to Triethylamine Density
This guide aims to provide a complete and detailed overview of triethylamine density. We will explore the fundamental concepts, measurement techniques, influencing factors, and practical implications associated with this critical property.
By delving into the intricacies of TEA density, this resource will empower professionals and students alike to:
- Gain a thorough understanding of TEA's physical behavior.
- Apply density measurements effectively in various applications.
- Ensure safe and efficient handling and storage practices.
Ultimately, this guide serves as a practical resource for anyone working with triethylamine, promoting informed decision-making and best practices in the laboratory and beyond.
Triethylamine's widespread use can be attributed to its unique chemical properties. It readily accepts protons, making it an effective base in organic reactions. It also serves as a catalyst, accelerating reaction rates without being consumed itself.
Moreover, TEA acts as a valuable building block in the synthesis of complex molecules, enabling the creation of novel materials and pharmaceuticals. Its applications span diverse fields, including:
Pharmaceuticals: Acting as a catalyst and intermediate in drug synthesis. Chemical Synthesis: Serving as a base and solvent in various reactions. Rubber Processing: Contributing to the production of high-quality rubber products. Corrosion Inhibition: Protecting metal surfaces from degradation.
What is Triethylamine (TEA)? A Chemical Profile
To fully grasp the nuances of triethylamine density, it is crucial to first establish a clear understanding of its fundamental chemical properties. This foundation will allow us to better appreciate the factors influencing its density and its behavior in various applications.
Triethylamine: Definition and Properties
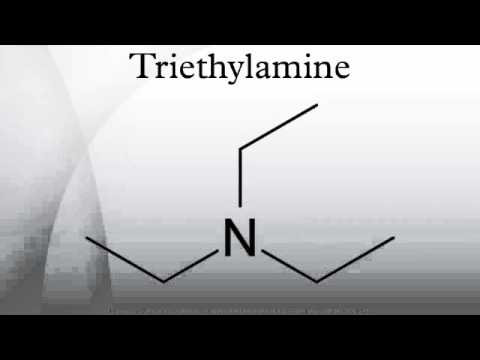
Triethylamine (TEA), also known as N,N-diethylethanamine, is a tertiary amine with the chemical formula (C2H5)3N. It is a volatile, colorless liquid at room temperature and possesses a strong, fishy, ammonia-like odor.
TEA is a key organic base widely employed in chemical synthesis and various industrial processes. Its basicity stems from the lone pair of electrons on the nitrogen atom, allowing it to readily accept protons.
Some of its basic physical properties include:
- Appearance: Colorless liquid
- Odor: Strong, fishy, ammonia-like
- Boiling point: 88.8 °C (191.8 °F; 361.9 K)
- Melting point: -114.7 °C (-174.5 °F; 158.5 K)
- Density: ~0.726 g/mL at 20 °C
Chemical Formula and Structure of TEA
The chemical formula for triethylamine is C6H15N. This formula indicates that each molecule of TEA consists of six carbon atoms, fifteen hydrogen atoms, and one nitrogen atom.
Structurally, TEA comprises a central nitrogen atom bonded to three ethyl groups (-C2H5). This specific arrangement is what defines it as a tertiary amine.
The three ethyl groups sterically hinder the nitrogen atom to some extent, which impacts its reactivity and selectivity in chemical reactions.
Molecular Weight Significance
The molecular weight of triethylamine is approximately 101.19 g/mol. Molecular weight is a crucial physical property, as it directly influences various aspects of a compound's behavior.
For instance, it determines the molar concentration in solutions. Also affects vapor pressure and boiling point.
Understanding the molecular weight is essential for stoichiometric calculations in chemical reactions, allowing for precise control over reaction ratios and yields.
CAS Number: A Unique Identifier
The Chemical Abstracts Service (CAS) number for triethylamine is 121-44-8. This number serves as a unique and unambiguous identifier for this specific chemical substance.
CAS numbers are invaluable for:
- Database searches
- Regulatory compliance
- Safety information retrieval
Using the CAS number ensures that you are referring to the correct chemical compound, regardless of variations in nomenclature or trade names. This is particularly important in safety data sheets (SDS) and other regulatory documents.
Triethylamine's behavior hinges on its inherent properties, but to truly harness its potential, understanding its density is essential. This parameter governs how TEA interacts within a system, influencing everything from reaction kinetics to storage stability.
Deciphering Triethylamine Density: A Deep Dive
Density, a fundamental physical property, provides crucial insights into the behavior of substances. It essentially describes how much "stuff" is packed into a given space. Understanding density is not merely academic; it is essential for the safe and efficient handling, storage, and utilization of triethylamine.
Defining Density and its Units
Density is defined as mass per unit volume. The most common units are grams per milliliter (g/mL) and kilograms per cubic meter (kg/m³). For triethylamine, density dictates how it will layer with other liquids, how much mass you are handling per unit volume, and the precision required in dispensing it.
Density is not constant. It varies with external conditions, making it a dynamic rather than static property.
Key Factors Affecting Density: Temperature and Pressure
The density of triethylamine, like any liquid, is sensitive to changes in temperature and pressure. These two factors play a significant role in determining the density value and must be carefully considered in any application.
Temperature and Triethylamine Density
Temperature has a direct inverse relationship with density. As temperature increases, the kinetic energy of the molecules increases. This increased energy causes molecules to move further apart, leading to an expansion in volume.
Since density = mass/volume, an increase in volume leads to a decrease in density. This phenomenon is known as thermal expansion.
For TEA, this means that its density will be lower at higher temperatures and higher at lower temperatures. This must be considered in chemical reactions where temperature is a factor, as it affects the concentration of TEA.
Pressure and Triethylamine Density
Pressure also influences density, although to a lesser extent for liquids compared to gases. Increasing pressure forces molecules closer together, decreasing the volume and thereby increasing the density.
The effect of pressure on liquids is typically small due to their relative incompressibility. However, when dealing with high-pressure applications or highly sensitive measurements, pressure's impact on TEA density should be taken into account.
While TEA is not highly compressible, changes should be considered when performing advanced calculations or high-pressure applications.
The Importance of Density in Applications
Density plays a critical role in various applications of triethylamine, impacting reaction rates, process efficiency, and overall quality control.
Chemical Reactions
In chemical reactions, density directly influences the concentration of reactants. Accurate knowledge of TEA density is essential for precise stoichiometry and reaction optimization. Using incorrect density values can lead to inaccurate measurements, impacting yield and product purity.
Process Optimization
In industrial processes, density is crucial for process monitoring and control. Density measurements can be used to track the progress of a reaction, ensure consistent product quality, and optimize separation processes. Variations in density can signal deviations from expected parameters, prompting corrective actions.
Quality Control
Density serves as a key quality control parameter for triethylamine. Measuring the density of incoming raw materials or final products helps verify purity and consistency. Deviations from specified density ranges can indicate contamination or degradation, triggering further analysis and quality assurance measures.
Deciphering the density of triethylamine requires more than just theoretical understanding; it demands proficiency in practical measurement techniques.
Measuring Triethylamine Density: Methods and Best Practices
Accurate density determination is crucial for quality control, reaction optimization, and ensuring the safe handling of triethylamine (TEA). Several methods exist for measuring density, each with its own advantages and limitations. This section will explore common techniques, emphasizing best practices for obtaining reliable results.
Common Measurement Techniques
The choice of method often depends on the desired level of accuracy, the available equipment, and the volume of sample available. Three widely used techniques are pycnometry, hydrometry, and the use of digital density meters.
The Pycnometer Method
The pycnometer method, also known as the specific gravity bottle method, is a classic and highly accurate technique for determining liquid density.
It involves precisely measuring the mass of a known volume of liquid at a specific temperature.
Procedure
- The clean, dry pycnometer is first weighed empty.
- It is then filled with deionized water, ensuring no air bubbles are trapped, and weighed again. This step determines the pycnometer's exact volume.
- The pycnometer is then emptied, thoroughly dried, and filled with triethylamine.
- The filled pycnometer is weighed.
- The density of triethylamine is calculated by dividing the mass of the triethylamine by the volume of the pycnometer (determined from the water measurement).
Considerations
- Temperature control is critical. Pycnometers are usually calibrated for use at a specific temperature (typically 20°C), and measurements should be performed as close to this temperature as possible or corrected for temperature variations.
- The pycnometer must be scrupulously clean and dry to avoid any contamination that could affect the accuracy of the measurement.
- Care must be taken to avoid air bubbles, which can lead to errors in volume determination.
The Hydrometer Method
The hydrometer method offers a simpler and quicker alternative to pycnometry, although it is generally less precise.
A hydrometer is a weighted glass instrument that floats in a liquid, with a calibrated scale indicating the liquid's specific gravity or density.
Procedure
- A clean, dry hydrometer is gently lowered into a sample of triethylamine in a graduated cylinder or similar container.
- The hydrometer should float freely without touching the sides or bottom of the container.
- The density is read directly from the hydrometer scale at the point where the liquid surface intersects the scale.
Considerations
- The hydrometer must be appropriate for the density range of triethylamine.
- The liquid must be free of air bubbles or suspended particles that could affect the hydrometer's buoyancy.
- The hydrometer reading should be taken at eye level to avoid parallax errors.
- Hydrometers are usually calibrated for a specific temperature and temperature corrections may be necessary.
Digital Density Meters
Digital density meters offer a rapid and automated method for density determination.
These instruments use an oscillating U-tube that vibrates at a specific frequency. The frequency of oscillation changes depending on the density of the liquid filling the tube.
Procedure
- A small sample of triethylamine is injected into the instrument's measuring cell.
- The instrument automatically measures the oscillation frequency and calculates the density.
- The density is displayed digitally on the instrument's screen.
Considerations
- Digital density meters offer high precision and accuracy, but they require regular calibration using certified reference materials.
- The instrument must be properly maintained and cleaned to ensure accurate measurements.
- The sample must be free of particulate matter that could interfere with the oscillation of the U-tube.
- Temperature control is usually integrated into the instrument and should be verified.
Practical Considerations for Accurate Density Measurements
Regardless of the method used, several practical considerations are crucial for achieving accurate and reliable density measurements:
- Temperature Control: Density is highly temperature-dependent. Maintaining a constant and known temperature throughout the measurement process is essential. Using a temperature-controlled water bath or a digital density meter with integrated temperature control is recommended.
- Calibration: Regularly calibrate instruments, such as pycnometers and digital density meters, using certified reference materials. This ensures the accuracy and traceability of measurements.
- Sample Preparation: Ensure the triethylamine sample is representative of the bulk material and is free from contaminants, air bubbles, or suspended particles that could affect the density measurement. Degassing the sample may be necessary.
- Proper Technique: Follow the specific procedures for each measurement method carefully. Consistent technique minimizes random errors and improves reproducibility.
- Multiple Measurements: Take multiple measurements and calculate the average value. This helps to identify and minimize random errors. The standard deviation of the measurements provides an indication of the precision of the results.
By carefully considering these factors and adhering to best practices, accurate and reliable density measurements of triethylamine can be obtained, leading to improved process control, product quality, and safety.
Deciphering the density of triethylamine requires more than just theoretical understanding; it demands proficiency in practical measurement techniques. Understanding how this property translates into real-world scenarios reveals the breadth of TEA's utility and underscores its significance across diverse fields.
Triethylamine in Action: Exploring its Applications
Triethylamine (TEA) isn't confined to laboratory experiments or academic discussions; it's a workhorse chemical that enables countless processes across various industries. Its versatility stems from its chemical properties, including its density, which directly impacts its behavior and effectiveness in different applications.
Pharmaceutical Prowess
In the pharmaceutical realm, triethylamine acts as a crucial catalyst and intermediate in the synthesis of numerous drug compounds.
As a catalyst, it facilitates reactions by accepting protons, speeding up the formation of desired products.
Its density is vital here, as it determines the volume needed for accurate dosing in reaction mixtures.
TEA can also be incorporated into the structure of drug molecules as an intermediate.
This requires precise control over its concentration, directly related to its density, to ensure the final product meets stringent quality standards.
Chemical Synthesis Cornerstone
Triethylamine's role in chemical synthesis extends far beyond pharmaceuticals. It serves as a versatile base, scavenging acids and promoting desired reactions.
Its relatively low density makes it easy to handle and dispense accurately in laboratory settings.
Furthermore, TEA functions as a solvent, dissolving reactants and facilitating interactions between them.
The density of TEA impacts its miscibility with other solvents, influencing reaction rates and yields.
Beyond the Beaker: Diverse Industrial Applications
Triethylamine finds applications in industries seemingly unrelated to pharmaceuticals or fine chemical synthesis.
In rubber processing, it acts as an accelerator in the vulcanization process, improving the strength and elasticity of rubber products.
It also plays a role as a corrosion inhibitor in various industrial systems.
Its density is a factor in determining its effectiveness in forming protective layers on metal surfaces.
Furthermore, it helps to control the viscosity and flow characteristics of the formulations in which it is used.
Density's Decisive Role: Influencing Performance and Efficiency
The density of triethylamine is not merely a physical constant; it's a critical parameter that directly influences its performance and efficiency in all its applications.
Accurate knowledge of TEA's density allows for precise dosing and concentration control in chemical reactions.
This is essential for achieving optimal yields and minimizing waste.
Density also affects the mixing and transport properties of TEA, influencing its distribution within a reaction mixture or industrial system.
In applications where TEA is used as a solvent, its density influences its ability to dissolve other compounds, thereby affecting reaction rates and overall process efficiency.
By carefully considering and controlling the density of triethylamine, engineers and chemists can optimize processes, improve product quality, and ensure the safe and effective use of this versatile chemical.
Safety First: Handling and Storage of Triethylamine
Having explored the diverse applications of triethylamine (TEA), it's paramount to shift our focus to the critical aspects of safety. TEA, while a valuable asset in various industries, demands strict adherence to safety protocols during handling and storage. Neglecting these precautions can lead to hazardous situations, impacting both human health and the environment.
The Imperative of Safety Precautions
Handling triethylamine requires a proactive and informed approach to safety. It's not merely about following guidelines; it's about understanding the inherent risks and implementing measures to mitigate them. A culture of safety must be cultivated within any environment where TEA is used, ensuring that all personnel are adequately trained and equipped.
This begins with a thorough understanding of TEA's potential hazards. TEA is a flammable liquid and a strong irritant. Exposure can cause severe burns to the skin, eyes, and respiratory system. Inhalation of high concentrations can lead to dizziness, nausea, and even unconsciousness. Therefore, appropriate personal protective equipment (PPE) is essential.
Deciphering the Material Safety Data Sheet (MSDS)
The Material Safety Data Sheet (MSDS), now often referred to as a Safety Data Sheet (SDS), is an indispensable resource for anyone working with TEA. It's the definitive guide to understanding the chemical's properties, hazards, and safe handling procedures. Think of it as the first line of defense against potential accidents.
Hazard Identification and Risk Assessment
The MSDS clearly outlines the potential hazards associated with TEA exposure. It details the acute and chronic health effects, flammability risks, and reactivity concerns. A thorough review of this section allows for a comprehensive risk assessment, enabling the implementation of appropriate control measures.
First Aid Measures: Immediate Response
In the event of an accidental exposure, the MSDS provides critical first aid instructions. This includes procedures for skin and eye contact, inhalation, and ingestion. Knowing these measures beforehand can significantly reduce the severity of the injury and potentially save lives. Time is of the essence in such situations.
Firefighting Procedures: Containing the Threat
TEA's flammability poses a significant fire risk. The MSDS outlines appropriate firefighting techniques, including the use of specific extinguishing agents. Water may be ineffective and could potentially spread the fire. Understanding these procedures is crucial for containing a TEA-related fire safely and effectively.
Accessing MSDS Resources
MSDS documents for triethylamine are readily available from various sources. Chemical suppliers typically provide them with the product. Online databases, such as those maintained by chemical safety organizations, also offer access to comprehensive MSDS information. It is crucial to consult the MSDS specific to the TEA product being used, as formulations and concentrations can vary. Some reliable resources include:
- Sigma-Aldrich: (Merck) Offers MSDS documents for various grades of triethylamine.
- Fisher Scientific: Provides access to TEA MSDS for their products.
Optimal Storage Conditions: Preserving Quality and Density
Proper storage is essential for maintaining the quality and stability of TEA, including its density. Incorrect storage can lead to degradation, contamination, and increased hazards. Adhering to specific guidelines ensures the chemical remains safe and effective for its intended use.
Temperature Control: Preventing Degradation
TEA should be stored in a cool, dry, and well-ventilated area. Excessive heat can accelerate degradation and increase the risk of fire. The ideal storage temperature is typically between 15°C and 25°C (59°F and 77°F).
Container Integrity: Preventing Leaks and Contamination
TEA should be stored in tightly sealed, non-reactive containers. Glass or stainless steel are generally suitable materials. Avoid containers made of materials that may react with TEA, such as certain plastics. Regularly inspect containers for signs of damage or leakage.
Ventilation: Minimizing Vapor Exposure
Adequate ventilation is crucial for preventing the buildup of TEA vapors in storage areas. TEA has a pungent odor, and prolonged exposure to vapors can cause respiratory irritation. A well-ventilated area helps to maintain a safe and comfortable working environment.
Segregation: Avoiding Incompatible Materials
TEA should be stored separately from incompatible materials, such as strong oxidizers, acids, and halogenated compounds. Mixing TEA with these substances can result in violent reactions. Proper segregation minimizes the risk of accidental contact and potential hazards.
Video: Triethylamine Density: The Ultimate Guide You Must See
Frequently Asked Questions About Triethylamine Density
Here are some common questions about triethylamine density and its applications, based on our guide.
What exactly is triethylamine density and why is it important?
Triethylamine density is a measure of how much triethylamine is packed into a given volume. It's usually expressed in grams per milliliter (g/mL) or kilograms per cubic meter (kg/m³). Knowing the triethylamine density is crucial for accurate measurement, dispensing, and calculations in chemical reactions and industrial processes.
How does temperature affect triethylamine density?
Generally, as temperature increases, triethylamine density decreases. This is because the molecules move faster and take up more space. It's important to consider the temperature at which the triethylamine density is measured, as it can significantly impact accuracy.
What is the typical triethylamine density value?
The triethylamine density typically falls around 0.728 g/mL at 20°C. However, it is always recommended to consult a reliable source, like a chemical supplier's specification sheet, for the precise triethylamine density at a specific temperature for the batch you are using.
How can I accurately measure triethylamine density?
You can accurately measure triethylamine density using tools like a pycnometer, hydrometer, or a digital density meter. Be sure to calibrate your equipment and follow proper procedures to minimize errors. Also, ensure the triethylamine sample is at a known and stable temperature during measurement.