Oxygen Ion Charge: The Shocking Truth You Need To Know!
Understanding electronegativity is crucial; Pauling's scale effectively quantifies an atom's ability to attract electrons. Linus Pauling significantly contributed to our understanding of chemical bonds, and his work directly illuminates why oxygen readily forms ions. The fundamental property of an oxygen atom that leads to this ionic behavior is the fact that an oxygen ion has a charge of -2, resulting from its tendency to gain two electrons to achieve a stable octet configuration. Therefore, considering electronegativity, the efforts of Linus Pauling, and the oxygen atom's quest for stability, we will explore this intriguing an oxygen ion has a charge of -2 and its implications.
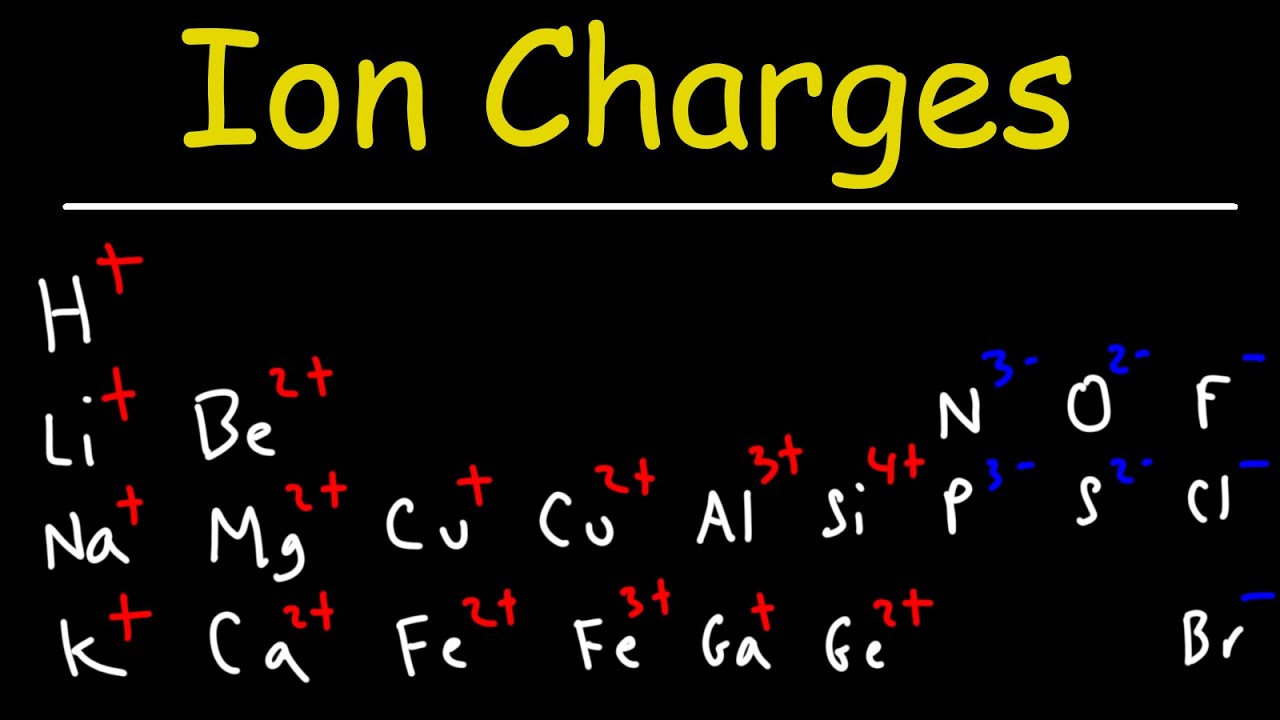
Image taken from the YouTube channel The Organic Chemistry Tutor , from the video titled How To Determine The Charge of Elements and Ions - Chemistry .
Chemistry is built upon understanding the interactions of atoms.
At the heart of these interactions lies the concept of the ion, an atom or molecule that has either gained or lost electrons.
This seemingly simple gain or loss has profound consequences, dictating how elements bond, the types of compounds they form, and the properties of the world around us.
But what exactly is an ion?
What are Ions?
Ions are atoms or molecules that carry an electrical charge.
This charge arises from an imbalance between the number of positively charged protons in the nucleus and the negatively charged electrons orbiting it.
A neutral atom has an equal number of protons and electrons, resulting in no net charge.
However, when an atom gains electrons, it becomes negatively charged and is called an anion.
Conversely, if an atom loses electrons, it becomes positively charged and is called a cation.
The magnitude of the charge depends on the number of electrons gained or lost.
The Curious Case of Oxygen: Why a -2 Charge?
Our specific focus is on oxygen.
Why does an oxygen ion almost always carry a -2 charge (O²⁻)?
This isn't an arbitrary assignment.
It's rooted in oxygen's electronic structure and a fundamental principle called the octet rule.
Unlocking the answer to this question provides crucial insight into the behavior of oxygen.
The Importance of Understanding Oxygen's Ionic Charge
Understanding why oxygen forms an ion with a -2 charge is not merely an academic exercise.
It’s essential for comprehending a vast range of chemical phenomena.
From the formation of oxides, which are ubiquitous in the Earth's crust, to the intricate workings of biological systems, oxygen's ionic behavior plays a central role.
This knowledge is crucial for understanding chemical bonds, predicting compound formation, and appreciating the properties of countless materials that shape our world.
Chemistry is built upon understanding the interactions of atoms. At the heart of these interactions lies the concept of the ion, an atom or molecule that has either gained or lost electrons. This seemingly simple gain or loss has profound consequences, dictating how elements bond, the types of compounds they form, and the properties of the world around us. But what exactly is an ion?
What are Ions?
To truly grasp the behavior of oxygen and its -2 charge, we must first establish a firm understanding of what an ion actually is.
Defining the Ion
An ion, at its core, is an atom or molecule that carries an electrical charge.
This charge isn't inherent to the element itself, but rather a consequence of an imbalance.
Specifically, an imbalance between the number of positively charged protons residing within the atom's nucleus and the negatively charged electrons orbiting that nucleus.
A perfectly neutral atom exists when these two forces are in equilibrium.
The Dance of Electrons: Gaining and Losing
The formation of an ion is a direct result of an atom either gaining or losing electrons.
It's important to remember that protons are tightly bound within the nucleus.
Therefore, elements do not gain or lose protons under normal chemical conditions.
The gain or loss of electrons is the mechanism by which an atom transforms into an ion.
Anions and Cations: A Tale of Two Charges
Ions aren't simply "charged atoms"; they are distinctly classified based on the nature of their charge: negative or positive.
This distinction gives rise to two fundamental types of ions: anions and cations.
Anions: The Negatively Charged
An anion is an ion that carries a negative charge.
This negative charge is a direct consequence of the atom gaining one or more electrons.
With more negatively charged electrons than positively charged protons, the atom takes on a net negative charge.
Cations: The Positively Charged
Conversely, a cation is an ion that carries a positive charge.
This positive charge arises from the atom losing one or more electrons.
By losing electrons, the atom now has more positively charged protons than negatively charged electrons.
Oxygen: A Fundamental Element
Having established the foundational principles of ion formation, it's time to turn our attention to the element at the heart of this discussion: oxygen. Understanding oxygen's fundamental nature, its properties, and its place in the periodic table is crucial to comprehending why it readily forms an ion with a -2 charge.
Oxygen is far more than just another entry on the periodic table. It is a cornerstone of life as we know it and a key player in countless chemical processes.
The Breath of Life and Chemical Reactions
Oxygen's designation as a fundamental element stems from its indispensable role in both biological and chemical realms. It is, quite literally, the breath of life, essential for respiration in most living organisms, facilitating the conversion of nutrients into energy.
Beyond respiration, oxygen is a powerful oxidizing agent, driving a wide array of chemical reactions, from combustion to corrosion. Its ability to readily accept electrons makes it a highly reactive element.
Key Properties of Oxygen
Oxygen exhibits a set of characteristic properties that dictate its behavior and reactivity. As a nonmetal, oxygen lacks the metallic luster and conductivity of metals. It exists as a gas at room temperature, typically as a diatomic molecule (O₂).
Its high electronegativity is a particularly important characteristic, indicating its strong tendency to attract electrons in chemical bonds. This property directly contributes to its propensity to form negative ions.
Oxygen's Place in the Periodic Table
Oxygen resides in Group 16 (also known as the chalcogens) of the periodic table. Its position within this group provides valuable insights into its chemical behavior.
Elements in the same group share similar valence electron configurations. Like other chalcogens, oxygen has six valence electrons, predisposing it to gain two more electrons to achieve a stable octet configuration.
The elements in Group 16 exhibit a trend of increasing metallic character down the group. Oxygen, being at the top, is decidedly nonmetallic and the most reactive of its group members, after fluorine.
Having explored oxygen's properties and its position within the periodic table, we now need to delve into the specifics of its atomic structure. This detailed look at the atomic number and electron configuration of oxygen is vital, because it provides the fundamental understanding we need to explain why it readily forms an ion with a -2 charge.
Atomic Number and Electron Configuration of Oxygen
The behavior of an atom is dictated by its structure, and to understand why oxygen behaves the way it does, we must first examine its atomic number and electron configuration.
Oxygen's Atomic Number: A Definition of Identity
Every element is defined by its atomic number.
This number represents the quantity of protons found in the nucleus of an atom of that element.
Oxygen has an atomic number of 8.
This signifies that every oxygen atom contains eight protons within its nucleus.
The number of protons is immutable for a given element; changing the number of protons transforms the atom into a completely different element.
Neutrality and Electron Count
In its neutral state, an atom possesses an equal number of protons and electrons.
Since oxygen has 8 protons, a neutral oxygen atom will also have 8 electrons.
These electrons orbit the nucleus in specific energy levels or shells.
It is the arrangement of these electrons that dictates how an atom interacts with other atoms and forms chemical bonds.
Electron Configuration: Filling the Orbitals
The electron configuration describes the arrangement of electrons within these energy levels and sublevels (orbitals).
This arrangement follows specific rules, dictated by quantum mechanics.
For oxygen, the electron configuration is 1s² 2s² 2p⁴.
Let's break this down:
-
1s²: The innermost energy level (n=1) has one s orbital, which can hold a maximum of two electrons. Oxygen completely fills this orbital.
-
2s²: The second energy level (n=2) has one s orbital, which is also filled with two electrons in oxygen.
-
2p⁴: The second energy level also has three p orbitals, each capable of holding two electrons, for a total capacity of six. Oxygen has four electrons in these p orbitals.
Significance of the 2p⁴ Configuration
The 2p⁴ configuration is key to understanding oxygen's reactivity.
It indicates that oxygen has a partially filled outermost electron shell (also known as the valence shell).
This incomplete shell makes oxygen highly reactive, driving it to seek ways to achieve a more stable electron configuration.
The drive for stability ultimately leads to the formation of ions, which we will discuss in the following sections.
Having laid the foundation by examining oxygen’s atomic number and electron configuration, we can now understand the underlying reasons why it forms a -2 ion. This involves exploring the concept of valence electrons and the compelling octet rule, which dictates the behavior of atoms as they seek stability.
Valence Electrons and the Octet Rule: The Drive for Stability
The chemical behavior of an element is intimately linked to the arrangement of electrons in its outermost shell. These electrons, known as valence electrons, are the key players in chemical bonding. Understanding their role is crucial to grasping how atoms interact and form molecules.
Defining Valence Electrons
Valence electrons are defined as the electrons residing in the outermost electron shell of an atom. These are the electrons farthest from the nucleus. And they are the ones most readily available to participate in chemical reactions. They determine an atom's bonding behavior.
It's essential to distinguish valence electrons from core electrons, which are those occupying the inner shells and are less involved in bonding. The number of valence electrons an atom possesses dictates how it will interact with other atoms.
Oxygen's Six Valence Electrons
Looking back at oxygen's electron configuration (1s² 2s² 2p⁴), we can identify its valence electrons. The outermost shell is the second shell (n=2). It contains both the 2s and 2p sublevels. Oxygen has 2 electrons in its 2s sublevel and 4 electrons in its 2p sublevel.
This gives oxygen a total of six valence electrons. This number is critical because it dictates how oxygen will interact with other atoms to achieve stability.
The Octet Rule: A Quest for Completion
The octet rule is a fundamental principle in chemistry. It states that atoms tend to gain, lose, or share electrons in order to achieve a full outer shell containing eight electrons. This configuration mimics the stable electron arrangement of the noble gases. It results in minimal energy and maximum stability for the atom.
Atoms "want" to have eight valence electrons because this configuration is associated with high stability. Elements with incomplete valence shells are reactive and will readily form bonds to achieve an octet.
For example, noble gases like Neon and Argon are exceptionally stable because their valence shells already contain eight electrons (an octet), making them largely unreactive. The desire to attain this noble gas configuration drives much of chemical bonding.
Having established that oxygen possesses six valence electrons, and understanding the driving force of the octet rule, it becomes clear that oxygen is not already in its most stable state. To achieve this stability, oxygen must somehow acquire two more electrons to complete its valence shell. This inherent "need" is what dictates oxygen's path to ionic form.
Oxygen's Path to Ionic Form: Gaining Electrons
The driving force behind oxygen's transformation into an ion is its quest for stability, as dictated by the octet rule.
Atoms strive to have a full outermost electron shell, typically containing eight electrons.
Since oxygen possesses only six valence electrons, it exhibits a strong tendency to gain electrons rather than lose them.
This tendency sets the stage for understanding how oxygen becomes an ion with a negative charge.
The Electron Acquisition Process
Oxygen's journey to ionic form is characterized by the acquisition of two electrons.
These electrons are drawn to the partially filled valence shell of the oxygen atom due to the electromagnetic force.
Once oxygen gains these two negatively charged particles, it disrupts the initial balance between protons and electrons.
This imbalance is crucial in understanding oxygen’s ionic charge.
Formation of the Oxide Anion (O²⁻)
The act of gaining two electrons transforms the neutral oxygen atom into an anion, specifically the oxide ion, represented as O²⁻.
The superscript "2-" signifies that the ion has a charge of negative two.
This notation clearly communicates the ionic state of oxygen after the electron gain.
The oxide ion has a stable electron configuration, resembling that of the noble gas neon.
Resulting Charge of -2
The acquisition of two negatively charged electrons by a neutral oxygen atom results in a net charge of -2.
This is because the number of electrons now exceeds the number of protons in the nucleus by two.
The resulting negatively charged ion (O²⁻) is significantly more stable than the neutral oxygen atom.
This -2 charge is a fundamental property of the oxygen ion and dictates its interactions with other elements.
The stable O²⁻ anion readily forms chemical bonds with cations, leading to the creation of a vast array of compounds crucial to our world.
Having established that oxygen possesses six valence electrons, and understanding the driving force of the octet rule, it becomes clear that oxygen is not already in its most stable state. To achieve this stability, oxygen must somehow acquire two more electrons to complete its valence shell. This inherent "need" is what dictates oxygen's path to ionic form.
The previous sections detailed how oxygen achieves this stability by gaining two electrons, resulting in a -2 charge. But what are the broader ramifications of this seemingly simple ionic transformation? It’s not merely a matter of charge; it’s the key to understanding a vast array of chemical compounds and their properties.
The Significance of a -2 Charge: Oxides and Ionic Bonding
The oxide ion (O²⁻), with its characteristic -2 charge, is a cornerstone of inorganic chemistry. This charge is not just a number; it's a fundamental property that dictates how oxygen interacts with other elements to form a myriad of compounds, most notably oxides.
Formation of Oxide Compounds
Oxides are chemical compounds that contain at least one oxygen atom as well as one other element.
The -2 charge of the oxide ion is crucial in the formation of these compounds. Because of its charge, oxygen can readily bond with other ions.
Take, for example, magnesium oxide (MgO).
Magnesium (Mg) readily loses two electrons to achieve a stable electron configuration, becoming a Mg²⁺ ion. The oxide ion (O²⁻) then forms an ionic bond with the magnesium ion, resulting in the stable compound MgO.
Similarly, iron oxide (Fe₂O₃), commonly known as rust, forms when iron atoms lose electrons to oxygen atoms, creating iron ions (Fe³⁺) that bond with oxide ions.
The specific ratio of iron to oxygen is dictated by the need to balance the charges and create a neutral compound. Two Fe³⁺ ions (total charge +6) are required to balance three O²⁻ ions (total charge -6).
The Role of Ionic Bonds
The -2 charge of the oxide ion is the principal reason for the formation of ionic bonds with positively charged cations.
Ionic bonds arise from the electrostatic attraction between oppositely charged ions.
The strong electrostatic attraction between O²⁻ and cations results in compounds with distinctive properties, such as high melting points and the ability to conduct electricity when dissolved in water.
The strength of an ionic bond is directly influenced by the magnitude of the charges involved. The -2 charge on the oxide ion contributes to strong, stable ionic bonds.
Examples and Importance of Oxygen-Containing Compounds
Oxygen ions are prevalent in countless compounds essential to various fields, from geology to biology.
Silicon dioxide (SiO₂), or silica, is a primary component of sand and quartz, forming the backbone of many rocks and minerals.
Calcium oxide (CaO), also known as lime, is a crucial ingredient in cement and is used extensively in construction.
Water (H₂O), although not an ionic compound, demonstrates oxygen's ability to form stable bonds with other elements. It is essential for life. While oxygen is covalently bonded to hydrogen in water molecules, water's bent molecular shape and oxygen's high electronegativity contribute to its polar nature and its capacity to form hydrogen bonds, critical for many biological processes.
The prevalence and diversity of oxygen-containing compounds underscore the critical role of the oxide ion and its -2 charge in shaping the chemical world around us. Understanding this fundamental concept unlocks a deeper appreciation of the structure, properties, and reactivity of countless substances that impact our daily lives.
It’s not merely a matter of charge; it’s the key to understanding a vast array of chemical compounds and their properties. The interaction between magnesium and oxygen serves as a single example of this -2 dance. But there are more subtle influences that determine whether oxygen will form that -2 ion in the first place.
Factors Influencing Oxygen Ion Formation: Electronegativity
While the octet rule provides a compelling reason for oxygen to gain two electrons, it’s not the only factor at play. Several considerations determine whether oxygen will actually form an ion and participate in ionic bonding. These include the ionization energy of the other reacting atom and, perhaps most significantly, electronegativity.
The Role of Electronegativity
Electronegativity is a measure of an atom's ability to attract shared electrons in a chemical bond. It's a relative scale, with values generally increasing as you move from left to right and bottom to top on the periodic table (fluorine is the most electronegative element).
Oxygen is a highly electronegative element, meaning it has a strong pull on electrons.
Electronegativity and Oxygen's Affinity for Electrons
Oxygen's high electronegativity is directly related to its tendency to gain electrons and form a -2 ion.
When oxygen encounters an element with significantly lower electronegativity, the electronegativity difference between the two is high.
Oxygen will "win" the tug-of-war for electrons, effectively stripping them from the other atom to complete its octet. This electron transfer results in the formation of an oxide ion (O²⁻) and a positively charged ion (cation) of the other element.
Quantifying Electronegativity Difference
Linus Pauling developed the most common electronegativity scale. In his scale, Fluorine is assigned a value of 3.98, and oxygen is 3.44.
Consider the electronegativity difference between oxygen (3.44) and magnesium (1.31).
The large difference suggests that oxygen will readily pull electrons away from magnesium, leading to the formation of MgO.
However, if oxygen were to interact with fluorine (3.98), a different type of bond would most likely form due to the even higher electronegativity of fluorine.
Beyond Simple Ionic Bonds
It's important to note that electronegativity differences are not always so clear-cut.
When the electronegativity difference between oxygen and another element is small, a polar covalent bond may form instead of a full ionic bond.
In a polar covalent bond, electrons are shared unequally, resulting in partial charges on the atoms involved. This contrasts with ionic bonds, where electrons are essentially transferred completely.
Water (H₂O) is a prime example. Oxygen is more electronegative than hydrogen, so the shared electrons spend more time near the oxygen atom. This gives the oxygen a partial negative charge (δ-) and the hydrogen atoms partial positive charges (δ+), leading to water's polar nature and unique properties.
Ionization Energy Considerations
While electronegativity is a dominant factor, the ionization energy of the other element also plays a role.
Ionization energy is the energy required to remove an electron from an atom.
Elements with low ionization energies readily lose electrons, making them more likely to form ionic bonds with oxygen.
In summary, while the octet rule explains why oxygen seeks to gain electrons, electronegativity and ionization energy help determine how and with whom it will achieve this stability, dictating the type of chemical bond formed and the resulting properties of the compound.
Video: Oxygen Ion Charge: The Shocking Truth You Need To Know!
Oxygen Ion Charge: FAQs
Here are some frequently asked questions to help clarify the concept of oxygen ion charge and its significance.
What exactly is an oxygen ion?
An oxygen ion is simply an oxygen atom that has gained or lost electrons, resulting in a net electrical charge. This is different from a neutral oxygen atom, which has an equal number of protons and electrons.
What is the typical charge of an oxygen ion?
In most cases, when oxygen forms an ion, it gains two electrons. Because it gains two negatively charged electrons an oxygen ion has a charge of -2. This is because oxygen atoms have a strong tendency to achieve a stable electron configuration.
Why does oxygen usually form a -2 ion?
Oxygen has six electrons in its outermost electron shell. To achieve a stable, full outer shell, it needs two more electrons. Gaining these two electrons results in a negative two (-2) charge, making it an oxide ion.
Where are oxygen ions commonly found?
Oxygen ions, particularly oxide ions (O²⁻), are found in a wide range of compounds, including metal oxides like rust (iron oxide), many minerals, and even in biological systems playing crucial roles in energy production.
