Oxygen & Fire: Unlocking the Secrets of Combustion!
Fire Tetrahedron, a model illustrating the four elements needed for fire, clearly highlights heat, fuel, and a chemical chain reaction, but often underemphasizes the crucial significance of oxygen. The National Fire Protection Association (NFPA), dedicated to fire safety, provides resources that consistently underscore the role of oxygen in combution and spread of fire. Understanding the process through techniques like Computational Fluid Dynamics (CFD) allows scientists to simulate fire behavior and analyze the impact of oxygen concentration. Moreover, the research of Antoine Lavoisier, a pioneer in combustion studies, laid the foundation for understanding the oxidation process, revealing how oxygen's presence is not merely a component, but an active participant in sustaining and propagating flames.

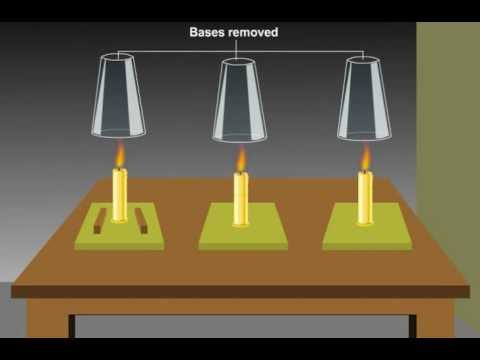
Image taken from the YouTube channel Easy To Learn , from the video titled Role of Oxygen in Combustion Process .
Fire, a captivating dance of light and heat, has held humanity's attention since the dawn of time. But what exactly fuels this phenomenon? The answer, at its core, lies in a fundamental relationship with another essential element: oxygen. This seemingly simple connection unlocks a world of complex chemical processes, revealing the science behind combustion.
This exploration delves into the heart of this relationship, illuminating the intricate mechanisms that govern fire's existence. Understanding this interplay is not merely an academic exercise; it's a crucial step towards ensuring safety, fostering innovation, and appreciating the powerful forces that shape our world.
Fire: Combustion Made Visible
Fire is not an entity in itself but rather a visible manifestation of combustion. It's the radiant outcome of a rapid chemical reaction that releases energy in the form of heat and light. Imagine a bonfire crackling on a cold night or the controlled burn within an engine. Both are visual representations of combustion, a process fundamentally driven by the interaction of fuel and an oxidizer.
The Indispensable Role of Oxygen
Among the various oxidizers that exist, oxygen reigns supreme as the most common and readily available. It acts as the essential ingredient, the catalyst that allows fuel to undergo rapid oxidation. Without a sufficient supply of oxygen, combustion simply cannot occur. This is why smothering a fire cuts off its oxygen source, effectively extinguishing the flames. Oxygen's presence is not merely supportive; it's foundational to the very existence of fire.
Why This Matters: Applications Across Disciplines
The understanding of oxygen's role in combustion extends far beyond basic curiosity. It is a cornerstone of knowledge applicable to a multitude of fields:
- Everyday Safety: Grasping the principles of combustion is critical for fire prevention and safe handling of flammable materials in homes and workplaces.
- Industrial Processes: Industries ranging from power generation to manufacturing rely on controlled combustion for energy production and material processing.
- Scientific Research: The study of combustion fuels advancements in areas like engine design, alternative fuels, and even space exploration.
- Environmental Science: Understanding combustion helps in mitigating the environmental impacts of wildfires and developing cleaner burning technologies.
A Roadmap to Understanding Combustion
This exploration will guide you through the core concepts that define the relationship between oxygen and fire:
- Defining the Core Components: We will start with clear definitions of oxygen, combustion, and fire, establishing a solid foundation for further exploration.
- The Science of Oxidation: We'll delve into the chemical process of oxidation, exploring its critical role in releasing energy during combustion.
- Ignition and Oxidizers: We will discuss the concepts of ignition temperature and oxidizers, highlighting their importance in initiating and sustaining fire.
- The Fire Tetrahedron: Finally, we will look at the fire tetrahedron, a comprehensive model that includes the crucial chemical chain reaction necessary for fire to persist.
Fire's dependence on oxygen is undeniable, but to truly grasp the essence of this fiery dance, we must first establish a solid foundation of understanding. This begins with clearly defining the key players involved: oxygen, combustion, and fire itself. Understanding the nuances of each term and their interconnectedness is crucial for navigating the science of flames.
Defining the Core Components: Oxygen, Combustion, and Fire
To unravel the complexities of fire, it is essential to define its fundamental components with clarity and precision. These definitions serve as the building blocks for a deeper comprehension of combustion.
The Breath of Fire: Defining Oxygen
Oxygen, denoted by the symbol O and atomic number 8, is a nonmetal element that exists as a diatomic molecule (O2) under standard conditions. It constitutes approximately 21% of the Earth's atmosphere. Its presence is not just abundant, but also critical for a myriad of processes, but perhaps its most well-known role is as the essential oxidizer in combustion.
Without oxygen, most fuels would simply remain inert, unable to release their stored energy in the spectacular display we call fire. Oxygen’s high electronegativity enables it to readily accept electrons from other substances. This forms the basis of the oxidation reactions that drive combustion.
Unveiling the Process: Defining Combustion
Combustion is a rapid, self-sustaining chemical process that involves the reaction between a substance with an oxidant, usually oxygen, to produce heat and light. It's a type of oxidation reaction, where a fuel rapidly combines with an oxidizer, releasing energy in the form of heat and electromagnetic radiation.
Think of it as an energetic transformation, where the chemical bonds within the fuel and oxidizer are broken and reformed, resulting in the release of vast amounts of energy. This process is highly exothermic, meaning it releases heat, often resulting in a significant temperature increase and the emission of light in the visible spectrum. The products of combustion often include gases such as carbon dioxide and water vapor, along with other byproducts depending on the fuel's composition.
Fire: The Visible Manifestation of Combustion
Fire is the visible, tangible result of the combustion process. It's the luminous phenomenon we perceive when a substance undergoes rapid oxidation, releasing heat and light. It's not a substance in itself, but rather the observable effect of a chemical reaction.
The color and intensity of fire depend on several factors, including the type of fuel, the temperature of the reaction, and the presence of other elements. From the warm glow of a candle flame to the raging inferno of a wildfire, fire presents itself in diverse forms. Yet, its presence always signals the underlying process of combustion.
The Interwoven Relationship: Oxygen, Combustion, and Fire
The relationship between oxygen, combustion, and fire is a direct and intertwined one. Oxygen is an indispensable ingredient for combustion to occur. Combustion, in turn, is the process that produces fire. Without oxygen, combustion cannot happen, and without combustion, there is no fire.
Imagine a three-legged stool, where oxygen, fuel, and heat are the legs. Remove any one of them, and the stool collapses. Similarly, removing oxygen from the equation will immediately extinguish the fire. Understanding this fundamental relationship is the first step in mastering the science of fire.
The Science of Oxidation: A Deeper Dive
With a clear understanding of oxygen, combustion, and fire established, we can now explore the underlying chemical processes that govern these phenomena. The engine driving fire is oxidation, a fundamental concept that extends far beyond the simple burning of materials. Understanding oxidation is key to understanding combustion.
Unpacking Oxidation: The Essence of Electron Transfer
Oxidation is fundamentally a chemical reaction characterized by the loss of electrons by a molecule, atom, or ion. It's a cornerstone concept in chemistry, playing a crucial role in diverse processes ranging from rusting to respiration.
While the term "oxidation" historically referred to reactions involving oxygen, its definition has evolved to encompass any reaction where electrons are transferred away from a substance. This broader understanding reveals oxidation's pervasive influence.
Fuels: The Heart of the Reaction
In the context of combustion, a fuel is any substance capable of undergoing rapid oxidation to produce heat and light. Common fuels include wood, propane, methane, and gasoline, all rich in chemical energy stored within their bonds.
Fuels serve as the electron donors in the oxidation process, readily surrendering electrons to an oxidizer (typically oxygen). This electron transfer releases energy in the form of heat and light, sustaining the combustion reaction.
Heat: The Catalyst for Combustion
Heat is a form of energy characterized by the movement of atoms or molecules within a substance. In combustion, heat plays a vital role in initiating and sustaining the reaction.
Reaching the ignition temperature is crucial. This is the minimum temperature required for a fuel to begin rapid, self-sustaining combustion.
The initial heat input provides the energy needed to break chemical bonds within the fuel molecules, allowing them to react with the oxidizer. The subsequent oxidation reactions release even more heat, creating a positive feedback loop that sustains the fire.
The Oxidative Cascade: Fuel's Transformation into Flame
The process of fuel oxidation is a captivating transformation where chemical energy is converted into light and thermal energy. It begins with the fuel interacting with an oxidizer.
The fuel molecules then begin surrendering electrons to the oxidizer. This electron transfer releases energy in the form of heat, raising the temperature of the surrounding gases.
The heated gases then begin to emit light, producing the visible flames we associate with fire. The rapid release of energy in the form of heat and light is what defines combustion as a dynamic process.
Heat acts as the catalyst, jumpstarting the reaction by providing the necessary energy to break the chemical bonds within the fuel. But how much heat is required, and what role do other substances play in ensuring a sustained blaze?
Ignition and Oxidizers: Fueling the Flame
Combustion, as we've established, is a rapid oxidation process. However, this process doesn't spontaneously occur. It requires specific conditions to initiate and sustain itself. Two key factors are ignition temperature and the presence of an oxidizer. These elements are crucial for “fueling the flame” and understanding how fire begins and persists.
Ignition Temperature: The Spark of Combustion
Every combustible material has a specific ignition temperature: the minimum temperature to which it must be heated in order to begin self-sustained combustion. This temperature represents the point where the molecules of the fuel gain enough energy to overcome the activation energy barrier for the oxidation reaction.
Think of it like pushing a boulder uphill.
The ignition temperature is the energy needed to get the boulder over the crest. Once over, it rolls down the other side on its own.
The ignition temperature varies significantly depending on the substance. For example, paper has a relatively low ignition temperature compared to wood. This explains why paper catches fire more easily.
Factors such as the fuel's physical state (solid, liquid, or gas), surface area, and the presence of catalysts can also affect the ignition temperature.
Oxidizers: The Oxygen Enablers
While heat provides the initial spark, an oxidizer is what allows the fire to burn. An oxidizer is a substance that accepts electrons from the fuel during oxidation.
While oxygen is the most common oxidizer, other substances like chlorine and fluorine can also act as oxidizers.
Oxidizers are not fuels themselves, but they are essential for combustion. They enable the fuel to rapidly release its stored energy in the form of heat and light. Without a sufficient supply of an oxidizer, combustion will quickly cease.
The Fire Triangle: A Foundation of Understanding
The fire triangle is a classic model representing the three essential elements needed for a fire to exist: fuel, heat, and oxygen (as an oxidizer). This simple diagram illustrates the interdependent nature of these components.
If any one of these elements is removed, the fire will be extinguished.
For instance, removing the fuel source (like turning off a gas valve) starves the fire. Removing the heat (like dousing with water) cools the fuel below its ignition temperature. And removing the oxygen (like smothering with a blanket) deprives the fire of the oxidizer it needs to continue.
The fire triangle serves as a foundational concept in fire safety.
Initiating Combustion: A Chain Reaction
The process of starting a fire involves raising a fuel to its ignition temperature in the presence of an oxidizer. Once the fuel reaches this temperature, the oxidation reaction begins, releasing heat and light.
This heat, in turn, further heats the fuel, sustaining the reaction.
This creates a self-sustaining chain reaction, as long as fuel and an oxidizer are available. Understanding how this chain reaction works is crucial for both preventing and extinguishing fires.
Heat acts as the catalyst, jumpstarting the reaction by providing the necessary energy to break the chemical bonds within the fuel. But how much heat is required, and what role do other substances play in ensuring a sustained blaze?
Ignition and Oxidizers: Fueling the Flame
Combustion, as we've established, is a rapid oxidation process. However, this process doesn't spontaneously occur. It requires specific conditions to initiate and sustain itself. Two key factors are ignition temperature and the presence of an oxidizer. These elements are crucial for “fueling the flame” and understanding how fire begins and persists.
The fire triangle, with its focus on fuel, heat, and oxygen, provides a foundational understanding of combustion. However, it presents a somewhat simplified view. A more comprehensive model, the fire tetrahedron, acknowledges a fourth crucial element: the chemical chain reaction. This addition provides critical insight into sustained combustion and effective fire suppression techniques.
The Fire Tetrahedron: A More Complete Model
The fire tetrahedron expands upon the fire triangle by adding a fourth element: the uninhibited chemical chain reaction.
This model provides a more accurate representation of how fires actually burn and offers advanced strategies for fire suppression.
The chemical chain reaction is a complex series of reactions that release heat, which in turn sustains the combustion process.
Understanding the Chemical Chain Reaction
At the molecular level, combustion involves a series of rapid reactions where fuel molecules break down and combine with oxygen.
These reactions release heat and generate free radicals, which are highly reactive atoms or molecules with unpaired electrons.
These free radicals collide with other fuel molecules, causing them to break down and react with oxygen.
This process generates more free radicals, perpetuating a self-sustaining chain reaction.
The Role of Inert Gases
Inert gases, such as nitrogen, argon, and helium, are chemically unreactive under normal conditions.
They do not readily participate in chemical reactions, including combustion.
Their primary role in fire suppression is to displace oxygen, effectively reducing the concentration of oxygen below the level necessary to sustain combustion.
This suffocating effect inhibits the chemical chain reaction and can extinguish the fire.
Examples of Inert Gas Use
Inert gases are commonly used in fire suppression systems in enclosed environments, such as data centers and museums.
These systems quickly release a large volume of inert gas, reducing the oxygen concentration and extinguishing the fire without damaging sensitive equipment or artifacts.
Interrupting the Chain Reaction: A Key to Extinguishment
The chemical chain reaction is crucial for sustaining a fire.
Therefore, interrupting this chain reaction is an effective method of fire suppression.
Certain fire suppressants, such as halons (now largely phased out due to environmental concerns) and some newer chemical agents, work by interfering with the free radicals involved in the chain reaction.
These agents effectively neutralize the free radicals, slowing down or stopping the combustion process.
How Chain Reaction Inhibition Works
These suppressants introduce molecules that react with the free radicals, stabilizing them and preventing them from reacting with fuel molecules.
This effectively breaks the chain reaction, causing the fire to diminish and eventually extinguish.
Understanding the role of the chemical chain reaction, and methods to interrupt it, is key to developing effective fire suppression strategies and technologies.
Video: Oxygen & Fire: Unlocking the Secrets of Combustion!
Frequently Asked Questions About Combustion
Here are some frequently asked questions about combustion to further your understanding of this fundamental chemical process.
What is the essential ingredient for fire?
Oxygen is the essential ingredient for fire. Without a sufficient supply of oxygen, combustion cannot occur. This highlights the role of oxygen in combustion and spread of fire.
What happens during the process of combustion?
During combustion, a rapid chemical reaction occurs between a substance (fuel) and an oxidant, usually oxygen. This reaction releases heat and light. The role of oxygen in combustion and spread of fire is crucial as it supports the oxidation of the fuel.
Why are some materials more flammable than others?
The flammability of a material depends on its chemical composition, surface area, and ignition temperature. Some materials readily react with oxygen at lower temperatures, making them easier to ignite and sustain the role of oxygen in combustion and spread of fire.
How does fire spread after it starts?
Fire spreads through a combination of heat transfer mechanisms: conduction, convection, and radiation. These mechanisms heat surrounding materials to their ignition temperature, allowing the combustion process to continue, driven by the role of oxygen in combustion and spread of fire.