Ionic vs Molecular: The SHOCKING Key Differences!
Chemical bonds, fundamental interactions that hold atoms together, manifest distinct characteristics depending on their nature. Electronegativity, a measure of an atom's ability to attract electrons, greatly influences bond type. Specifically, differences in electronegativity dictate whether a bond leans towards ionic or molecular. Considering this, researchers at MIT are constantly pushing boundaries. Molecular compounds typically exhibit lower melting and boiling points, showcasing properties different from ionic compounds. A tool such as ChemDraw helps illustrate these differences. Distinguishing ionic vs molecular properties is thus crucial for understanding material behavior.
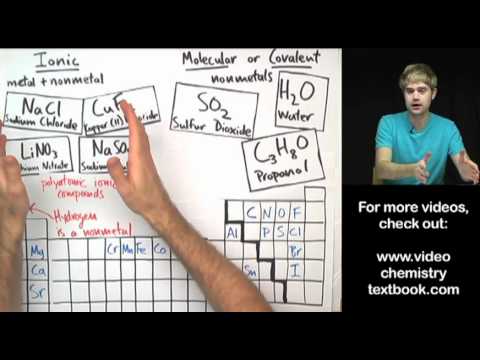
Image taken from the YouTube channel Tyler DeWitt , from the video titled Ionic vs. Molecular .
Chemistry, at its core, is the study of matter and its properties. Understanding the building blocks of matter – how atoms combine and interact – is fundamental to grasping chemical phenomena. Among the most important distinctions in chemistry is that between ionic compounds and molecular compounds.
These two classes of compounds, while both formed from atoms, exhibit strikingly different properties and behaviors. These differences stem from the distinct ways in which their constituent atoms are held together.
Why This Distinction Matters
The importance of understanding the differences between ionic and molecular compounds cannot be overstated. These compounds form the basis of countless substances we encounter daily, from the salt we sprinkle on our food to the water we drink. Their distinct properties dictate their uses and behaviors in various applications.
Ionic and molecular compounds play different and critical roles in biological systems, industrial processes, and environmental interactions. A solid foundation in the principles governing their formation and properties is therefore essential for anyone studying or working in chemistry, biology, materials science, or related fields.
Previewing Our Exploration
This article will embark on a comprehensive exploration of the key differences between ionic and molecular compounds. Our journey will focus on three fundamental aspects: their formation, properties, and behavior.
We will delve into the underlying mechanisms of chemical bonding. From the complete transfer of electrons in ionic bonding, to the sharing of electrons in covalent bonding. We will examine how these bonding differences give rise to the unique structural characteristics of each compound type.
Furthermore, we will compare their physical properties, such as melting and boiling points, and their electrical conductivity. We will explore their solubility in different solvents. Finally, we will illustrate how these differences manifest in real-world applications.
Defining Ionic Compounds: Electron Transfer and Electrostatic Attraction
As we begin to unravel the distinctions between ionic and molecular compounds, it’s crucial to first establish a clear understanding of what defines each. Here, we focus on ionic compounds, exploring their fundamental characteristics and the mechanisms behind their formation.
What are Ionic Compounds?
Ionic compounds are substances formed through the complete transfer of electrons from one atom to another. This transfer creates charged particles called ions.
Positively charged ions are called cations, and negatively charged ions are called anions.
These oppositely charged ions are then held together by strong electrostatic forces, forming what we know as an ionic bond.
Components of Ionic Compounds: Ionic Bonds
The very essence of an ionic compound lies in its ionic bonds. These are not the result of shared electrons, as we’ll see in molecular compounds.
Instead, ionic bonds arise from the powerful attraction between positive and negative ions.
This attraction is omnidirectional, meaning each ion is attracted to all surrounding ions of opposite charge, creating a vast, interconnected network.
This network gives rise to the characteristic properties of ionic compounds, such as their high melting points and brittleness.
Characteristics of Ionic Bonds: Emphasizing Electron Transfer
The key characteristic of ionic bonds is the complete transfer of electrons. One atom essentially "gives up" one or more electrons to another.
This transfer typically occurs between a metal and a nonmetal.
The metal atom loses electrons to become a positively charged cation, while the nonmetal atom gains electrons to become a negatively charged anion.
This transfer is driven by the quest for stability. Atoms gain or lose electrons to achieve a full outer electron shell, mimicking the stable electron configurations of noble gases.
Formation of Ionic Compounds: The Role of Electronegativity
The formation of ionic compounds is heavily influenced by electronegativity, which measures an atom's ability to attract electrons in a chemical bond.
Ionic compounds are most likely to form when there's a significant difference in electronegativity between the participating atoms.
Electronegativity Differences and Ion Formation
A large electronegativity difference indicates that one atom has a much stronger pull on electrons than the other.
This disparity leads to the complete transfer of electrons, resulting in the formation of ions.
For instance, sodium chloride (NaCl), common table salt, is formed when sodium (Na) and chlorine (Cl) react.
Chlorine is far more electronegative than sodium, pulling sodium's lone valence electron to itself, thus creating Na+ and Cl- ions which then form an ionic bond.
Resulting Ions: Cations and Anions
The atom that loses electrons becomes a cation, bearing a positive charge.
The magnitude of the charge corresponds to the number of electrons lost. For example, sodium (Na) loses one electron to form Na+, while magnesium (Mg) loses two to form Mg2+.
Conversely, the atom that gains electrons becomes an anion, carrying a negative charge.
The magnitude of the negative charge equals the number of electrons gained. For example, chlorine (Cl) gains one electron to become Cl-, while oxygen (O) gains two to form O2-.
The interplay of electronegativity and electron transfer is thus the engine driving the formation of ionic compounds.
As we've explored the world of ionic compounds and the electron transfer that defines them, a contrasting realm exists where atoms achieve stability not by giving away electrons, but by sharing them. This brings us to the fascinating world of molecular compounds.
Defining Molecular Compounds: Electron Sharing and Covalent Bonds
Molecular compounds represent a distinct class of chemical substances. Instead of relying on electrostatic attraction between ions, they arise from the sharing of electrons between atoms. This sharing leads to the formation of covalent bonds.
What are Molecular Compounds?
Molecular compounds, unlike their ionic counterparts, are formed when two or more atoms share electrons to achieve a stable electron configuration. This sharing of electrons results in the formation of a covalent bond, the fundamental building block of molecular substances.
These compounds are composed of discrete, individual molecules. Each molecule consists of a specific number of atoms held together by covalent bonds.
Covalent Bonds: The Essence of Molecular Compounds
Covalent bonds are the defining feature of molecular compounds. These bonds arise when atoms share one or more pairs of electrons. This mutual sharing allows each atom to achieve a more stable electron configuration, typically resembling that of a noble gas.
Unlike ionic bonds, which are omnidirectional, covalent bonds are highly directional. Meaning that they exist between specific atoms within the molecule.
This directionality dictates the shape and properties of the resulting molecule. The shared electrons reside primarily in the space between the bonded atoms, creating a region of high electron density that holds the atoms together.
Characteristics of Covalent Bonds
-
Electron Sharing: The cornerstone of covalent bonding. Atoms contribute electrons to be shared, achieving a more stable electron arrangement.
-
Directionality: Covalent bonds are directional, leading to specific molecular shapes. This is in stark contrast to the omnidirectional nature of ionic bonds.
-
Strength: Covalent bond strength can vary significantly depending on the atoms involved and the number of electron pairs shared (single, double, or triple bonds).
Formation of Molecular Compounds: Electronegativity and Electron Sharing
The formation of molecular compounds is intimately linked to the electronegativity differences between the participating atoms. Electronegativity is a measure of an atom's ability to attract electrons in a chemical bond.
Electronegativity's Role
When atoms with similar electronegativity values approach each other, neither atom has a strong enough pull to completely remove electrons from the other. Instead, they tend to share electrons to achieve stability.
This is in stark contrast to ionic compound formation, where a large electronegativity difference leads to complete electron transfer.
The Process of Electron Sharing
The process begins with the close approach of two atoms with comparable electronegativities. The valence electrons of each atom are attracted to the positively charged nucleus of the other.
As the atoms get closer, their electron clouds begin to overlap. The electrons become delocalized, meaning they are no longer exclusively associated with one atom but are shared between both.
This sharing of electrons lowers the overall energy of the system, leading to the formation of a stable covalent bond. The shared electrons spend most of their time in the region between the two nuclei, effectively holding the atoms together.
Types of Covalent Bonds
-
Single Bond: Formed when one pair of electrons is shared between two atoms.
-
Double Bond: Formed when two pairs of electrons are shared between two atoms, resulting in a stronger and shorter bond.
-
Triple Bond: Formed when three pairs of electrons are shared between two atoms, resulting in an even stronger and shorter bond.
As we've explored the world of ionic compounds and the electron transfer that defines them, a contrasting realm exists where atoms achieve stability not by giving away electrons, but by sharing them. This brings us to the fascinating world of molecular compounds.
Bonding: The Core Difference – Ionic vs. Covalent
The very essence of how atoms interact to form compounds lies in the nature of their chemical bonds. The stark contrast between ionic and covalent bonds dictates the fundamental properties and behaviors we observe in the macroscopic world. Understanding these differences is paramount to grasping the diverse chemistry that shapes our world.
Ionic Bonds: A Tale of Electron Transfer
Ionic bonds are born from a dramatic event: the transfer of electrons from one atom to another. Typically, this involves a metal atom readily surrendering one or more electrons to a nonmetal atom.
This electron transfer results in the formation of ions. Positively charged ions (cations) and negatively charged ions (anions) are formed.
The beauty of this lies in the electrostatic attraction between oppositely charged ions. This strong force holds the ions together in a rigid, three-dimensional lattice structure. This attraction is non-directional, meaning each ion is attracted to all neighboring ions of opposite charge.
This omnidirectional nature contributes to the characteristic properties of ionic compounds, such as high melting points and brittleness.
Covalent Bonds: The Art of Electron Sharing
In stark contrast to the decisive electron transfer of ionic bonds, covalent bonds arise from the sharing of electrons between two atoms. This sharing occurs when atoms have a similar affinity for electrons.
Neither atom is "strong" enough to completely wrest electrons away from the other. This mutual sharing allows each atom to achieve a more stable electron configuration, usually resembling that of a noble gas.
The shared electrons are primarily concentrated in the region between the two nuclei. This creates a strong, directional bond that holds the atoms together in a molecule. The nature of this sharing can range from equal sharing (nonpolar covalent) to unequal sharing (polar covalent), depending on the electronegativity differences between the atoms.
Electronegativity: The Guiding Force
The key to predicting the type of bond that will form between two atoms lies in understanding electronegativity.
Electronegativity is a measure of an atom's ability to attract electrons in a chemical bond. Atoms with high electronegativity have a strong pull on electrons, while atoms with low electronegativity have a weaker pull.
A large difference in electronegativity between two atoms (generally greater than 1.7 on the Pauling scale) will result in an ionic bond. The more electronegative atom will effectively "steal" the electron(s) from the less electronegative atom.
Conversely, a small difference in electronegativity (generally less than 0.4) will result in a covalent bond. The atoms will share the electrons, either equally or unequally, depending on the subtle differences in their electronegativity values.
Electronegativity serves as a predictor for the type of chemical bond that will be formed between atoms.
It's not always a clear-cut decision for what kind of bond is formed, as bond formation lies on a spectrum.
Understanding electronegativity and its influence on bonding allows us to predict the properties of various compounds.
As we've explored the world of ionic compounds and the electron transfer that defines them, a contrasting realm exists where atoms achieve stability not by giving away electrons, but by sharing them. This brings us to the fascinating world of molecular compounds.
Structure: Crystal Lattices vs. Discrete Molecules
The fundamental difference in bonding between ionic and molecular compounds profoundly dictates their structural arrangements in the macroscopic world.
Ionic compounds organize themselves into extended, three-dimensional networks, whereas molecular compounds exist as individual, distinct units.
The Ionic Embrace: Crystal Lattice Formation
Ionic compounds, born from electrostatic attraction, arrange themselves in a highly ordered and repeating pattern known as a crystal lattice.
This lattice is not simply a random jumble of ions; it's a carefully orchestrated arrangement where each ion is surrounded by ions of the opposite charge.
Imagine sodium chloride (NaCl), common table salt. Each sodium ion (Na+) is nestled among six chloride ions (Cl-), and conversely, each chloride ion is surrounded by six sodium ions.
This arrangement extends in three dimensions, creating a macroscopic crystal.
The strength of the electrostatic forces within this lattice is significant.
It requires a substantial amount of energy to overcome these forces and disrupt the lattice, explaining the high melting points of ionic compounds.
The crystal lattice structure also influences other properties, such as brittleness. If you apply enough force to shift the layers of ions, you bring ions of like charge into proximity, resulting in repulsion and ultimately, fracture.
The Molecular Realm: Discrete Entities
In stark contrast, molecular compounds are composed of discrete, individual molecules.
These molecules are formed when atoms share electrons through covalent bonds.
Unlike the extended network of ionic compounds, molecules exist as self-contained units with defined shapes and sizes.
Consider water (H2O). Each water molecule consists of two hydrogen atoms covalently bonded to a single oxygen atom.
These molecules exist independently of each other, held together by weaker intermolecular forces, not the strong electrostatic forces of the ionic lattice.
Shape and Size Matter
The shape of a molecule is critical to its function and properties.
The arrangement of atoms within a molecule is determined by the principles of valence shell electron pair repulsion (VSEPR) theory, which dictates that electron pairs around a central atom will arrange themselves to minimize repulsion.
This leads to a variety of molecular shapes, such as linear, bent, tetrahedral, and trigonal planar, each with unique properties.
The size of a molecule also influences its behavior. Larger molecules generally have stronger intermolecular forces and higher boiling points compared to smaller molecules.
The distinct nature of individual molecules, coupled with the variety of shapes and sizes they can adopt, gives molecular compounds a diverse range of properties and applications.
In the previous section, we explored how the fundamental nature of bonding dictates the structural organization of ionic and molecular compounds, with ionic compounds forming rigid crystal lattices and molecular compounds existing as discrete units. This difference in structure profoundly influences their physical properties, most notably their melting and boiling points.
Physical Properties: Melting Point and Boiling Point Comparison
Melting and boiling points serve as crucial indicators of the strength of the forces holding the constituent particles together. In this section, we'll dissect the contrasting thermal behaviors of ionic and molecular compounds, tying these differences back to the fundamental nature of their bonding and intermolecular forces.
The Thermal Stability of Ionic Compounds: A Consequence of Lattice Energy
Ionic compounds, characterized by their strong electrostatic interactions, exhibit remarkably high melting and boiling points.
This stems from the immense energy required to overcome the powerful attraction between the positively and negatively charged ions within the crystal lattice.
The lattice energy is the energy required to completely separate one mole of a solid ionic compound into its gaseous ions.
This value is directly proportional to the magnitude of the charges on the ions and inversely proportional to the distance between them.
Consequently, compounds with highly charged ions (e.g., MgO with Mg2+ and O2-) possess significantly higher lattice energies, and thus, melting and boiling points, than those with singly charged ions (e.g., NaCl with Na+ and Cl-).
Consider sodium chloride (NaCl), which melts at 801°C and boils at 1413°C.
These elevated temperatures reflect the substantial energy input needed to disrupt the extensive network of electrostatic attractions within its crystal lattice.
Disrupting the crystal lattice requires that enough energy is added so that the individual ions can overcome the attractive forces keeping them in place.
The Thermal Lability of Molecular Compounds: The Role of Intermolecular Forces
In stark contrast to ionic compounds, molecular compounds generally exhibit significantly lower melting and boiling points.
This is primarily due to the weaker nature of intermolecular forces (IMFs) that hold these molecules together in the solid and liquid states.
Unlike the strong electrostatic attractions in ionic compounds, IMFs are weaker attractive forces between molecules, not within them.
These forces arise from temporary or permanent dipoles within the molecules.
Types of Intermolecular Forces
Several types of IMFs exist, each with varying strengths:
-
London Dispersion Forces (LDF): Present in all molecules, these weak, temporary attractions arise from instantaneous fluctuations in electron distribution. The larger the molecule and the more electrons it has, the stronger its LDFs.
-
Dipole-Dipole Forces: Occur in polar molecules that have a permanent dipole moment.
-
Hydrogen Bonding: A particularly strong type of dipole-dipole interaction that occurs when hydrogen is bonded to highly electronegative atoms like oxygen, nitrogen, or fluorine.
Covalent Bond Strength
It is important to note that while IMFs determine melting and boiling points, covalent bond strength is a different factor.
Covalent bonds hold atoms together within a molecule and are generally much stronger than IMFs.
Breaking covalent bonds requires much higher energies and leads to chemical changes (decomposition), not just phase transitions.
Impact on Phase Transitions
The strength of the prevailing IMFs directly influences the energy required to induce phase transitions (melting and boiling).
For example, methane (CH4), a nonpolar molecule held together only by weak LDFs, has a melting point of -182.5°C and a boiling point of -161.5°C.
Water (H2O), on the other hand, exhibits significantly higher melting (0°C) and boiling (100°C) points due to the presence of strong hydrogen bonding.
The relatively strong hydrogen bonds between water molecules require significantly more energy to break, resulting in the higher temperatures needed for melting and boiling compared to methane.
In essence, the lower melting and boiling points of molecular compounds are a direct consequence of the weaker intermolecular forces that govern their interactions, requiring far less energy to overcome compared to the robust electrostatic forces in ionic lattices.
In the previous section, we dissected the contrasting thermal behaviors of ionic and molecular compounds, tying these differences back to the fundamental nature of their bonding and intermolecular forces. Just as the forces holding these compounds together dictate their response to heat, they also govern their ability to conduct electricity, a property that hinges on the presence and mobility of charge carriers.
Electrical Conductivity: Charge Carriers in Different Environments
Electrical conductivity is a material's ability to conduct electric current. This property is fundamentally linked to the availability of mobile charge carriers, be they electrons or ions, within the substance.
The contrasting bonding and structures of ionic and molecular compounds lead to distinct differences in their electrical conductivity.
Conductivity in Ionic Compounds: The Role of Mobile Ions
Ionic compounds in their solid state are notoriously poor conductors of electricity. The ions, held rigidly in the crystal lattice, are unable to move freely and carry charge.
However, this picture changes dramatically when ionic compounds are dissolved in a polar solvent like water or melted into a molten state.
Ionic Compounds in Aqueous Solution
When an ionic compound dissolves in water, the polar water molecules surround and separate the ions, a process called solvation.
These now-free ions, no longer constrained by the lattice structure, become mobile charge carriers.
The presence of these mobile ions allows the solution to conduct electricity effectively. The dissolved ions can move freely, carrying electrical charge from one electrode to another.
Molten Ionic Compounds
Similarly, when an ionic compound is heated to its melting point, the rigid crystal lattice breaks down.
The ions gain sufficient kinetic energy to overcome the electrostatic forces holding them in place.
As a result, the molten ionic compound contains mobile ions that can conduct electricity. This is why molten salts are used in certain industrial electrolytic processes.
Conductivity in Molecular Compounds: Limited Charge Carriers
In stark contrast to ionic compounds, molecular compounds are generally poor conductors of electricity in all states – solid, liquid, and dissolved in solution.
This is primarily because molecular compounds are composed of neutral molecules, not charged ions.
The Absence of Mobile Ions or Electrons
Covalent bonds involve the sharing of electrons between atoms, resulting in a neutral molecule with no free-moving charged particles.
Even in the liquid or dissolved state, most molecular compounds do not dissociate into ions.
Therefore, there are typically no mobile charge carriers available to facilitate electrical conductivity.
Exceptions to the Rule
While the vast majority of molecular compounds are non-conductive, there are exceptions. Certain molecular compounds, particularly acids in aqueous solution, can ionize to a small extent.
For instance, hydrochloric acid (HCl) dissolves in water to form hydronium ions (H3O+) and chloride ions (Cl-), which can then conduct electricity. However, the conductivity of these solutions is generally much lower than that of ionic solutions, as the concentration of ions is much lower.
Furthermore, some molecular compounds with delocalized pi electron systems, such as certain polymers and organic semiconductors, can exhibit some degree of electrical conductivity. However, this is a specialized area beyond the scope of this core comparison.
In summary, the presence of mobile ions is critical for electrical conductivity. Ionic compounds provide these mobile ions when dissolved or melted, while molecular compounds generally lack them, rendering them poor conductors of electricity.
In the previous section, we dissected the contrasting thermal behaviors of ionic and molecular compounds, tying these differences back to the fundamental nature of their bonding and intermolecular forces. Just as the forces holding these compounds together dictate their response to heat, they also govern their ability to conduct electricity, a property that hinges on the presence and mobility of charge carriers.
Solubility: Dissolving Ionic and Molecular Compounds
Solubility, the ability of a substance (solute) to dissolve in a solvent, is a crucial property governed by the interplay of intermolecular forces. The phrase "like dissolves like" succinctly captures the essence of solubility, highlighting the importance of matching the polarity of the solute and solvent. This section will explore how ionic and molecular compounds exhibit vastly different solubility behaviors in polar and nonpolar solvents, with a focus on water (a polar solvent) and organic solvents (typically nonpolar).
Solubility of Ionic Compounds
Ionic compounds, with their strong electrostatic interactions between ions, exhibit a pronounced affinity for polar solvents like water.
The Role of Water in Dissolving Ionic Compounds
Water molecules, being polar, can effectively solvate ions through ion-dipole interactions.
The partially negative oxygen atoms in water surround positive cations, while the partially positive hydrogen atoms surround negative anions.
This solvation process effectively reduces the interionic attractions within the crystal lattice, allowing the ions to disperse throughout the water.
The energy released during solvation, known as the hydration energy, must be sufficient to overcome the lattice energy of the ionic compound for dissolution to occur.
Insolubility in Nonpolar Solvents
In contrast, ionic compounds are generally insoluble in nonpolar solvents such as hexane or toluene.
These solvents lack the necessary partial charges to effectively interact with and solvate ions.
The weak London dispersion forces present in nonpolar solvents are insufficient to overcome the strong electrostatic attractions holding the ions together in the crystal lattice.
Solubility of Molecular Compounds
Molecular compounds exhibit a wider range of solubility behaviors depending on their polarity and intermolecular forces.
Polar Molecular Compounds
Polar molecular compounds, such as alcohols and sugars, tend to be soluble in polar solvents like water.
This solubility arises from the formation of hydrogen bonds or dipole-dipole interactions between the solute and solvent molecules.
For example, ethanol (C2H5OH) readily dissolves in water because it can form hydrogen bonds with water molecules through its hydroxyl (-OH) group.
Nonpolar Molecular Compounds
Nonpolar molecular compounds, such as hydrocarbons (e.g., methane, octane), are generally insoluble in water.
The only intermolecular forces present are London dispersion forces.
Water molecules are more attracted to each other via hydrogen bonding than to nonpolar molecules.
This disparity in intermolecular forces prevents effective mixing and leads to phase separation.
Solubility in Organic Solvents
Nonpolar molecular compounds readily dissolve in nonpolar organic solvents.
The London dispersion forces between the solute and solvent molecules are of comparable strength.
This allows them to interact favorably and mix homogeneously.
For instance, fats and oils (which are largely nonpolar) dissolve well in solvents like hexane or diethyl ether.
In the previous section, we dissected the contrasting thermal behaviors of ionic and molecular compounds, tying these differences back to the fundamental nature of their bonding and intermolecular forces. Just as the forces holding these compounds together dictate their response to heat, they also govern their ability to conduct electricity, a property that hinges on the presence and mobility of charge carriers. Now, shifting our focus from macroscopic properties to the very architecture of molecules, we turn to the tools that allow us to visualize and predict their three-dimensional shapes: Lewis structures and VSEPR theory.
Predicting Molecular Shape: Lewis Structures and VSEPR Theory
The three-dimensional shape of a molecule is not merely a cosmetic detail; it profoundly influences its physical and chemical properties.
Molecular shape dictates how a molecule interacts with other molecules, affecting everything from its melting point and boiling point to its reactivity and biological activity.
Understanding and predicting molecular shape requires the application of two key concepts: Lewis structures and Valence Shell Electron Pair Repulsion (VSEPR) theory.
Lewis Structures: Visualizing Molecular Architecture
Lewis structures are diagrams that represent the bonding between atoms in a molecule, as well as any lone pairs of electrons that may exist.
They provide a visual representation of the electron distribution within a molecule, acting as a crucial first step in predicting its shape.
To draw a Lewis structure, one must first determine the total number of valence electrons in the molecule.
Then, arrange the atoms, typically with the least electronegative atom in the center (except for hydrogen, which is always terminal).
Next, use pairs of electrons to form bonds between atoms, and distribute the remaining electrons as lone pairs to satisfy the octet rule (or duet rule for hydrogen).
If the central atom lacks an octet, multiple bonds (double or triple bonds) may be required.
Resonance structures, which represent multiple possible arrangements of electrons, may also need to be considered for some molecules.
VSEPR Theory: Electrons Behaving Badly (and Predictably)
VSEPR theory builds upon the foundation laid by Lewis structures.
It postulates that the shape of a molecule is determined by the repulsion between electron pairs surrounding the central atom.
These electron pairs, whether they are bonding pairs (involved in covalent bonds) or lone pairs (non-bonding), will arrange themselves to minimize repulsion and maximize the distance between them.
Applying VSEPR: From Electron Groups to Molecular Geometry
The first step in applying VSEPR theory is to count the number of electron groups around the central atom.
An electron group can be a single bond, a double bond, a triple bond, or a lone pair.
Each of these counts as one group regardless of how many electrons it contains.
The number of electron groups then determines the electron-pair geometry, which is the arrangement of electron groups around the central atom.
Common electron-pair geometries include linear (2 electron groups), trigonal planar (3 electron groups), tetrahedral (4 electron groups), trigonal bipyramidal (5 electron groups), and octahedral (6 electron groups).
The molecular geometry then describes the arrangement of only the atoms in the molecule, taking into account the presence of lone pairs.
Lone pairs exert a greater repulsive force than bonding pairs, which can distort the bond angles and affect the overall shape of the molecule.
For example, a molecule with four electron groups, two of which are lone pairs, will have a bent molecular geometry, even though its electron-pair geometry is tetrahedral.
Examples of Molecular Shapes Predicted by VSEPR
Consider carbon dioxide (CO2).
Its Lewis structure shows a central carbon atom double-bonded to two oxygen atoms.
There are two electron groups around the carbon atom, leading to a linear electron-pair geometry and, consequently, a linear molecular geometry.
Water (H2O), on the other hand, has a central oxygen atom bonded to two hydrogen atoms and bearing two lone pairs.
This gives it four electron groups, a tetrahedral electron-pair geometry, and a bent molecular geometry.
Ammonia (NH3) has a central nitrogen atom bonded to three hydrogen atoms and bearing one lone pair, which gives it four electron groups, a tetrahedral electron-pair geometry, and a trigonal pyramidal molecular geometry.
By carefully considering the number of electron groups and the influence of lone pairs, VSEPR theory provides a powerful tool for predicting the shapes of molecules, which in turn helps us to understand their properties and behavior.
In the previous section, we dissected the contrasting thermal behaviors of ionic and molecular compounds, tying these differences back to the fundamental nature of their bonding and intermolecular forces. Just as the forces holding these compounds together dictate their response to heat, they also govern their ability to conduct electricity, a property that hinges on the presence and mobility of charge carriers. Now, shifting our focus from macroscopic properties to the very architecture of molecules, we turn to the tools that allow us to visualize and predict their three-dimensional shapes: Lewis structures and VSEPR theory. But theoretical knowledge gains its true value when applied to the tangible world around us. From the table salt seasoning our food to the fuels powering our vehicles, ionic and molecular compounds are integral to countless aspects of daily life.
Real-World Examples: Ionic and Molecular Compounds in Action
Chemistry is not confined to laboratories; it's the foundation of the world we inhabit. Ionic and molecular compounds, with their distinct properties, play crucial roles in a vast array of applications. Examining specific examples illuminates the practical significance of understanding their differences.
Ionic Compounds: Cornerstones of Industry and Health
Ionic compounds, characterized by their strong electrostatic interactions and crystalline structures, find applications in diverse fields.
Sodium Chloride (NaCl): More Than Just Table Salt
Sodium chloride, commonly known as table salt, is perhaps the most ubiquitous ionic compound. Beyond its culinary uses as a flavor enhancer and preservative, it serves as a raw material in the production of chlorine gas, sodium hydroxide, and other essential chemicals.
It is also crucial for maintaining electrolyte balance in living organisms, facilitating nerve impulse transmission and muscle function.
Magnesium Oxide (MgO): From Antacids to Construction
Magnesium oxide boasts a high melting point and chemical stability, making it valuable in various industrial applications.
It's a key component of refractory materials used in high-temperature furnaces. In medicine, it is employed as an antacid to neutralize stomach acid and as a laxative.
Moreover, it finds use in construction materials, contributing to the fire resistance of cement and flooring.
Calcium Chloride (CaCl2): De-icing and Desiccation
Calcium chloride is widely used as a de-icing agent on roads and sidewalks during winter, effectively lowering the freezing point of water.
Its hygroscopic nature also makes it an effective desiccant, absorbing moisture from the air in various applications, including food preservation and dust control.
In medicine, it can be used to treat calcium deficiencies.
Molecular Compounds: Fueling Life and Technology
Molecular compounds, formed by the sharing of electrons, exhibit a wide range of properties that make them essential in biological processes, energy production, and everyday products.
Water (H2O): The Elixir of Life
Water, a seemingly simple molecule, is fundamental to all known life. Its polarity and ability to form hydrogen bonds enable it to act as a universal solvent, facilitating countless chemical reactions within living organisms.
It regulates temperature, transports nutrients, and participates directly in many metabolic processes. Its unique properties also make it indispensable in various industrial applications, from cooling systems to cleaning processes.
Methane (CH4): A Primary Energy Source
Methane is the principal component of natural gas, a major source of energy for heating, electricity generation, and transportation.
Its relatively simple molecular structure and high energy content per unit mass make it an efficient and widely used fuel. However, its combustion also contributes to greenhouse gas emissions, prompting research into more sustainable alternatives.
Glucose (C6H12O6): Fueling Biological Processes
Glucose, a simple sugar, is the primary source of energy for most living organisms. Through cellular respiration, glucose is broken down to produce ATP (adenosine triphosphate), the energy currency of cells.
It also serves as a building block for more complex carbohydrates, such as starch and cellulose, which play vital roles in energy storage and structural support.
In the food industry, glucose is used as a sweetener and preservative.
Video: Ionic vs Molecular: The SHOCKING Key Differences!
Ionic vs Molecular: Frequently Asked Questions
Got questions about ionic vs molecular compounds? Here are some quick answers to common queries.
What's the biggest difference between ionic and molecular compounds in terms of how they're formed?
Ionic compounds are formed through the transfer of electrons between atoms, creating ions (charged particles) that are then attracted to each other. Molecular compounds, on the other hand, are formed when atoms share electrons to achieve a stable electron configuration. This difference in electron behavior is crucial in understanding the properties of ionic vs molecular substances.
Why do ionic compounds generally have much higher melting points than molecular compounds?
The strong electrostatic forces between ions in an ionic lattice require a significant amount of energy to overcome. Molecular compounds are held together by weaker intermolecular forces. This difference in bond strength explains why ionic vs molecular compounds have drastically different melting points.
Are ionic compounds always soluble in water?
Not always. While many ionic compounds are soluble in water because water molecules are polar and can effectively solvate the ions, some ionic compounds are insoluble. The relative strength of the ion-ion attraction compared to the ion-water interaction determines the solubility of specific ionic vs molecular substances, some of which do not dissolve.
Do ionic or molecular compounds conduct electricity better?
Ionic compounds conduct electricity only when molten or dissolved in water. In these states, the ions are free to move and carry a charge. Molecular compounds generally do not conduct electricity because they don't readily form ions in solution. The presence of freely moving ions distinguishes ionic vs molecular electrical conductivity.
So there you have it! Hopefully, you now have a much clearer picture of the key differences between ionic vs molecular compounds. Chemistry can seem tricky, but breaking it down makes it much easier to understand!
