Fluorine Dot Structure: See It, Understand It, Master It
Understanding fluorine dot structure is fundamental to grasping molecular bonding principles taught in many chemistry courses. Gilbert N. Lewis, a pioneering figure in chemical bonding theory, introduced the concept of electron dot structures, a key tool for visualizing valence electrons. These structures, simplified representations of valence electrons, are now widely used, and fluorine dot structure can be generated using online tools, such as molecular visualization software. Mastering these structures allows one to predict molecular properties, particularly relevant in fields like pharmaceutical development.
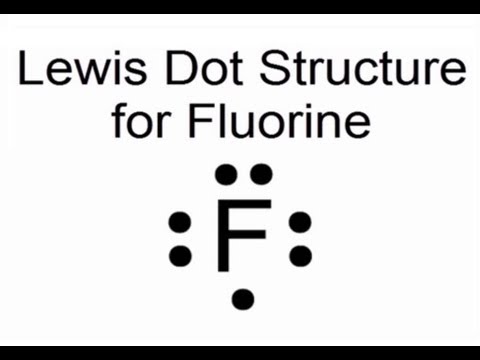
Image taken from the YouTube channel Wayne Breslyn (Dr. B.) , from the video titled Lewis Dot Structure for Fluorine Atom (F) .
Fluorine, symbolized as F, is a fascinating element.
It is known for its exceptional reactivity.
It holds a pivotal position in the world of chemistry.
But what makes it so reactive, and why is it so important?
This article aims to answer these questions.
We will provide a comprehensive guide.
This guide will lead you to understand and master the Lewis Dot Structure of Fluorine.
What This Guide Offers
Lewis Dot Structures are visual representations of an atom’s valence electrons.
These structures are crucial for understanding chemical bonding.
This guide will provide a step-by-step approach.
This approach will help you to confidently draw the Lewis Dot Structure for Fluorine.
We will also explore its role in various chemical compounds.
The Significance of Fluorine's Dot Structure
Understanding the Lewis Dot Structure of Fluorine is more than just a chemistry exercise.
It is a gateway to understanding fundamental chemical bonding principles.
Fluorine's dot structure unveils how and why it readily forms bonds with other elements.
This knowledge is critical for:
- Predicting molecular properties.
- Understanding chemical reactions.
- Designing new molecules and materials.
By understanding this seemingly simple representation, a whole world of chemical insights opens up.
So, let's begin our journey into the world of Fluorine and its Dot Structure!
Fluorine's dot structure unveils how and why it readily forms bonds with other elements, opening up a world of chemical insights.
So, let's begin our journey into the world of Fluorine and its Dot Structure! Before we delve into the intricacies of its Lewis Dot Structure, it's crucial to establish a solid foundation by understanding the fundamental properties that govern Fluorine's behavior.
Fluorine: An Element in Focus
Fluorine, the 9th element on the periodic table, is far more than just a symbol and a number. Its unique characteristics dictate its reactivity and its role in the chemical world. Understanding these properties is paramount to grasping why it behaves the way it does.
Basic Properties of Fluorine
Fluorine (F) exists as a pale yellow gas at room temperature.
It possesses a pungent, irritating odor that is detectable even at low concentrations.
Caution: Fluorine is highly toxic and corrosive, requiring extreme care when handling it.
Its diatomic form, F2, is the most common state in which it is found.
Fluorine's Position in the Periodic Table
Fluorine resides in Group 17 of the periodic table, also known as the halogens.
This placement offers insight into its chemical behavior.
Halogens are characterized by having seven valence electrons.
This makes them highly reactive nonmetals, eager to gain an electron to achieve a stable octet.
Other notable halogens include chlorine (Cl), bromine (Br), and iodine (I), each sharing similar tendencies to form negative ions.
The Significance of High Electronegativity
Fluorine stands out among all elements due to its exceptionally high electronegativity.
Electronegativity measures the ability of an atom to attract electrons towards itself in a chemical bond.
Fluorine's electronegativity is the highest on the Pauling scale (3.98).
This means it has an unparalleled ability to pull electrons away from other atoms during bond formation.
As a result, Fluorine readily forms strong, polar covalent bonds with other elements.
This leads to the formation of stable compounds with unique properties.
Its extreme electronegativity also contributes to its tendency to exist as a negative ion (F-) in ionic compounds.
This highlights the central role Fluorine plays in a multitude of chemical reactions.
Fluorine's exceptional electronegativity, arising from its position in the periodic table and its electronic configuration, sets the stage for its eagerness to engage in chemical bonding. But before we can fully appreciate how fluorine bonds, we need to understand the underlying principles that govern these interactions: valence electrons and the octet rule. These concepts are fundamental to understanding not just fluorine, but chemical bonding in general.
Valence Electrons and the Octet Rule: The Foundation of Bonding
The drive for atoms to form bonds stems from a fundamental desire for stability, a state of low energy. This quest for stability is intimately linked to the arrangement of electrons, particularly those in the outermost shell.
Understanding Valence Electrons
Valence electrons are the electrons residing in the outermost electron shell of an atom. These are the electrons primarily involved in chemical bonding. They are the "social butterflies" of the atomic world, interacting with other atoms to form molecules.
The number of valence electrons dictates an atom's bonding behavior. Atoms "want" to achieve a full outermost shell. This often means gaining, losing, or sharing electrons with other atoms.
Determining Fluorine's Valence Electrons
Fluorine (F) has an electron configuration of 1s²2s²2p⁵. This means it has two electrons in its innermost shell (1s²) and seven electrons in its outermost shell (2s²2p⁵).
Therefore, fluorine possesses seven valence electrons. This explains its high reactivity. It only needs one more electron to achieve a full outer shell. The periodic table is your friend. Elements in the same group have the same number of valence electrons.
The Octet Rule: Achieving Stability
The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a full outer shell containing eight electrons. This configuration mimics the stable electron arrangement of noble gases.
Fluorine, with its seven valence electrons, is a prime example of an element striving to satisfy the octet rule. It readily accepts one electron from another atom to complete its outer shell, forming a stable negative ion (F⁻) or participating in covalent bonds.
Limitations and Exceptions to the Octet Rule
While the octet rule is a useful guideline, it's important to acknowledge its limitations. It primarily applies to elements in the second period of the periodic table (like fluorine).
There are several exceptions. Some molecules feature atoms with fewer or more than eight electrons in their valence shell. For example, molecules like SF6 and ClF3 involve elements exceeding the octet rule.
However, for understanding the basic bonding behavior of fluorine, the octet rule serves as a valuable foundation. It highlights its strong tendency to gain one electron and form stable chemical bonds.
Fluorine's eagerness to gain just one more electron makes it a highly reactive element. Now, let’s translate this understanding of valence electrons and the octet rule into a visual representation: the Lewis Dot Structure. This structure will provide a clear picture of how fluorine's valence electrons are arranged and how it seeks to achieve a stable octet.
Step-by-Step: Drawing the Fluorine Lewis Dot Structure
The Lewis Dot Structure, also known as an electron dot diagram, is a simplified representation of an atom's valence electrons. It allows us to visualize how atoms form chemical bonds. For fluorine, understanding its Lewis Dot Structure is crucial for predicting its bonding behavior.
The Process of Depiction: A Visual Guide
Creating the Lewis Dot Structure for a single fluorine atom is a straightforward process:
-
Identify the Chemical Symbol: Begin by writing the chemical symbol for fluorine, which is simply F. This symbol represents the nucleus and all the core electrons (non-valence electrons) of the fluorine atom.
-
Determine the Number of Valence Electrons: As we established earlier, fluorine has seven valence electrons. This is the critical piece of information. These are the only electrons we will represent in the Lewis Dot Structure.
-
Represent Valence Electrons as Dots: Now, we will represent each valence electron as a dot surrounding the fluorine symbol. Imagine the symbol F at the center of a square. We will place dots around the symbol, following a specific pattern: one dot on each side of the square, before pairing them up.
-
Arrange the Dots: Start by placing a single dot on the top, right, bottom, and left sides of the F symbol. This accounts for four of the seven valence electrons. Then, begin pairing up the dots on each side until all seven are placed. You should end up with three sides having two dots each and one side with a single dot.
The Significance of Lone Pairs
In the Lewis Dot Structure of fluorine, you will notice that three pairs of dots are clustered together. These pairs are called lone pairs.
Lone pairs are pairs of valence electrons that are not involved in chemical bonding. They belong exclusively to the fluorine atom and contribute significantly to its chemical behavior.
Fluorine has three lone pairs because, out of its seven valence electrons, six are paired, leaving only one unpaired electron available for bonding. This unpaired electron is what drives fluorine to form a single bond with another atom, thereby completing its octet.
Visualizing Fluorine's Structure
The final Lewis Dot Structure for fluorine should look like this:
. .
. F .
:
Where F is the chemical symbol for fluorine, the single dot on the bottom represents the unpaired electron available for bonding, and the paired dots represent the three lone pairs. This visual representation encapsulates fluorine's electronic state and its inherent desire to form a chemical bond.
Fluorine's eagerness to gain just one more electron makes it a highly reactive element. Now, let’s translate this understanding of valence electrons and the octet rule into a visual representation: the Lewis Dot Structure. This structure will provide a clear picture of how fluorine's valence electrons are arranged and how it seeks to achieve a stable octet.
Fluorine in Action: Chemical Bonding Scenarios
Fluorine doesn't exist in isolation very often. Its strong pull for electrons dictates that it almost always seeks to form a chemical bond with other elements.
This section explores some typical bonding scenarios, highlighting its propensity to form single bonds to achieve that coveted octet configuration.
The Single Bond Imperative
Fluorine's electronic configuration leaves it just one electron short of a full outer shell. This drives its chemistry. As such, fluorine almost invariably forms a single covalent bond. This allows it to share an electron with another atom, thus completing its octet.
Its exceptionally high electronegativity – the highest of all elements – means it attracts electrons very strongly. This also contributes to single bond formation. It essentially grabs tightly onto that shared electron.
Common Bonding Examples
Let's examine a few common examples to illustrate fluorine's bonding behavior:
Hydrogen Fluoride (HF)
In hydrogen fluoride (HF), fluorine shares a single electron with a hydrogen atom. Hydrogen also needs one more electron to achieve its stable duplet. This single bond is highly polar because of fluorine's electronegativity. Fluorine hogs the shared electrons, giving it a partial negative charge and leaving hydrogen with a partial positive charge.
Carbon Tetrafluoride (CF4)
Carbon tetrafluoride (CF4) showcases fluorine's ability to form multiple single bonds. Here, one carbon atom bonds with four fluorine atoms. Each fluorine atom contributes one electron to form a single covalent bond with the central carbon. Carbon, with its four valence electrons, forms four single bonds with four fluorine atoms. Thus achieving an octet for both the carbon and each fluorine atom.
The Diatomic Fluorine Molecule (F2)
Even fluorine can bond with itself! Fluorine gas exists as diatomic molecules, F2. In this case, each fluorine atom shares one electron with the other.
Lewis Dot Structure of F2
The Lewis Dot Structure of F2 clearly demonstrates the sharing of electrons. Each fluorine atom starts with seven valence electrons. By sharing one electron, each achieves a complete octet. The two fluorine atoms are connected by a single line representing the covalent bond. The remaining six valence electrons on each fluorine appear as three lone pairs around each atom.
Fluorine in More Complex Molecules
Fluorine's bonding is also found in more complex and fascinating molecular structures. It appears in various organic and inorganic compounds. It often serves to modify the properties of the molecule through its electronegativity.
Consider examples like sulfur hexafluoride (SF6) or chlorine trifluoride (ClF3). These show that fluorine can also sometimes be present in compounds that don't necessarily obey the octet rule. However, it still sticks to its tendency to form single bonds. These are exceptions which challenge our established rules of element bonding.
Fluorine's relentless pursuit of a full valence shell dictates its bonding behavior, largely limiting it to single bonds. Now, let's put this knowledge to the test. The true measure of understanding lies in application.
Practice Makes Perfect: Mastering the Dot Structure
Drawing Lewis Dot Structures can seem daunting at first. However, with consistent practice and a keen eye for detail, you'll be constructing them with ease. This section is dedicated to solidifying your understanding of fluorine's dot structure through practical examples and helpful tips.
Fluorine-Containing Molecules: Practice Examples
Let's delve into some examples that showcase fluorine's bonding in different molecular contexts. These examples will not only reinforce the principles we've discussed but also introduce scenarios where the octet rule might be bent, but never broken.
Sulfur Hexafluoride (SF6): An Octet Rule Exception
Sulfur hexafluoride (SF6) presents an interesting case. Sulfur, the central atom, is surrounded by six fluorine atoms. This means sulfur accommodates more than eight electrons in its valence shell.
To draw the Lewis structure, first, calculate the total valence electrons: Sulfur (6) + 6 Fluorine (6 x 7) = 48 valence electrons. Position sulfur as the central atom and arrange the six fluorine atoms around it.
Connect each fluorine atom to the sulfur atom with a single bond. This accounts for 12 electrons (6 bonds x 2 electrons/bond). Distribute the remaining 36 electrons as lone pairs around the fluorine atoms (3 lone pairs each, for a total of 18 lone pairs).
SF6 is a stable molecule, which means the "octet rule" is more of a guideline than a rigid rule. Sulfur can be "hypervalent" because it is in period 3, meaning it can utilize its d-orbitals to have more than eight electrons around it.
Chlorine Trifluoride (ClF3): Another Exception
Chlorine trifluoride (ClF3) is another molecule where the central atom (chlorine) exceeds the octet.
Calculate valence electrons: Chlorine (7) + 3 Fluorine (3 x 7) = 28 valence electrons. Place chlorine in the center, surrounded by three fluorine atoms.
Form single bonds between chlorine and each fluorine. This uses 6 electrons. Distribute the remaining 22 electrons. Each fluorine gets three lone pairs (18 electrons total). Chlorine gets two lone pairs.
Note that chlorine has 10 electrons around it (3 bonding pairs and 2 lone pairs). This is permitted because chlorine is in the third period.
Tips and Tricks: Avoiding Common Pitfalls
Drawing Lewis Dot Structures accurately requires meticulous attention to detail. Here are some tips to help you avoid common mistakes:
- Double-Check Valence Electrons: Always start by accurately counting the total number of valence electrons for all atoms in the molecule. This is the most frequent source of error. Refer to the periodic table and each element's group number to verify.
- Electronegativity Considerations: Remember that fluorine is highly electronegative. Fluorine is almost always on the outside.
- Lone Pair Placement: Ensure each fluorine atom (in most cases) has three lone pairs to complete its octet after forming a single bond. Distribute the remaining electrons as lone pairs around the other atoms.
- Octet Exceptions: Recognize when exceptions to the octet rule might occur. Be mindful of central atoms from the third period and beyond. They can sometimes accommodate more than eight valence electrons.
- Formal Charge: If there are multiple ways to draw a Lewis structure, calculate the formal charge on each atom. The structure with the lowest formal charges is usually the most stable.
- Practice Regularly: The best way to master Lewis Dot Structures is through consistent practice. Work through various examples and don't be afraid to seek help when needed.
- Visual Aids: Use online resources and textbooks to visually confirm your structures. Comparing your work to established examples helps solidify your understanding.
Video: Fluorine Dot Structure: See It, Understand It, Master It
Fluorine Dot Structure FAQs
Here are some frequently asked questions to help you further understand the fluorine dot structure.
Why does fluorine only form one bond?
Fluorine has seven valence electrons, meaning it needs only one more electron to achieve a stable octet. Therefore, fluorine only forms one covalent bond to complete its outer shell. This is evident when drawing the fluorine dot structure.
How is the fluorine dot structure different from other halogens?
While all halogens have seven valence electrons and generally form one bond, the main difference lies in the number of electron shells. Fluorine, being in the second period, has fewer electron shells than chlorine, bromine, or iodine. The fluorine dot structure reflects only its valence electrons, just like the other halogens' Lewis structures.
What does the fluorine dot structure tell us about its reactivity?
The fluorine dot structure shows that fluorine has a strong attraction for electrons to complete its octet. This high electronegativity explains why fluorine is the most reactive nonmetal. It readily takes electrons from other atoms to form stable compounds.
Can fluorine have an expanded octet?
Unlike some other elements in the third period or beyond, fluorine cannot have an expanded octet. This is because fluorine has no available d-orbitals in its valence shell. The fluorine dot structure always represents a molecule with a maximum of eight electrons surrounding the fluorine atom.
